Abstract
Tumour metastasis is a complex process involving reciprocal interplay between cancer cells and host stroma at both primary and secondary sites, and is strongly influenced by microenvironmental factors such as hypoxia1. Tumour-secreted proteins play a crucial role in these interactions2,3,4,5 and present strategic therapeutic potential. Metastasis of breast cancer to the bone affects approximately 85% of patients with advanced disease and renders them largely untreatable6. Specifically, osteolytic bone lesions, where bone is destroyed, lead to debilitating skeletal complications and increased patient morbidity and mortality6,7. The molecular interactions governing the early events of osteolytic lesion formation are currently unclear. Here we show hypoxia to be specifically associated with bone relapse in patients with oestrogen-receptor negative breast cancer. Global quantitative analysis of the hypoxic secretome identified lysyl oxidase (LOX) as significantly associated with bone-tropism and relapse. High expression of LOX in primary breast tumours or systemic delivery of LOX leads to osteolytic lesion formation whereas silencing or inhibition of LOX activity abrogates tumour-driven osteolytic lesion formation. We identify LOX as a novel regulator of NFATc1-driven osteoclastogenesis, independent of RANK ligand, which disrupts normal bone homeostasis leading to the formation of focal pre-metastatic lesions. We show that these lesions subsequently provide a platform for circulating tumour cells to colonize and form bone metastases. Our study identifies a novel mechanism of regulation of bone homeostasis and metastasis, opening up opportunities for novel therapeutic intervention with important clinical implications.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
17 April 2023
This article has been retracted. Please see the Retraction Notice for more detail: https://doi.org/10.1038/s41586-023-06048-x
References
Chan, D. A. & Giaccia, A. J. Hypoxia, gene expression, and metastasis. Cancer Metastasis Rev. 26, 333–339 (2007).
Jin, L. et al. Differential secretome analysis reveals CST6 as a suppressor of breast cancer bone metastasis. Cell Res. 22, 1356–1373 (2012).
Erler, J. T. et al. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell 15, 35–44 (2009).
Kaplan, R. N. et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 438, 820–827 (2005).
Blanco, M. A. et al. Global secretome analysis identifies novel mediators of bone metastasis. Cell Res. 22, 1339–1355 (2012).
Coleman, R. E. & Rubens, R. D. The clinical course of bone metastases from breast cancer. Br. J. Cancer 55, 61–66 (1987).
Steeg, P. S. Tumor metastasis: mechanistic insights and clinical challenges. Nature Med. 12, 895–904 (2006).
Chi, J. T. et al. Gene expression programs in response to hypoxia: cell type specificity and prognostic significance in human cancers. PLoS Med. 3, e47 (2006).
Smid, M. et al. Subtypes of breast cancer show preferential site of relapse. Cancer Res. 68, 3108–3114 (2008).
Kang, Y. et al. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell 3, 537–549 (2003).
El-Haibi, C. P. et al. Critical role for lysyl oxidase in mesenchymal stem cell-driven breast cancer malignancy. Proc. Natl Acad. Sci. USA 109, 17460–17465 (2012).
Cox, T. R. et al. LOX-mediated collagen crosslinking is responsible for fibrosis-enhanced metastasis. Cancer Res. 73, 1721–1732 (2013).
Erler, J. T. et al. Lysyl oxidase is essential for hypoxia-induced metastasis. Nature 440, 1222–1226 (2006).
van de Vijver, M. J. et al. A gene-expression signature as a predictor of survival in breast cancer. N. Engl. J. Med. 347, 1999–2009 (2002).
Baker, A. M., Bird, D., Lang, G., Cox, T. R. & Erler, J. T. Lysyl oxidase enzymatic function increases stiffness to drive colorectal cancer progression through FAK. Oncogene 32, 1863–1868 (2012).
Boyle, W. J., Simonet, W. S. & Lacey, D. L. Osteoclast differentiation and activation. Nature 423, 337–342 (2003).
Garrett, I. R. et al. Oxygen-derived free radicals stimulate osteoclastic bone resorption in rodent bone in vitro and in vivo. J. Clin. Invest. 85, 632–639 (1990).
Bax, B. E. et al. Stimulation of osteoclastic bone resorption by hydrogen peroxide. Biochem. Biophys. Res. Commun. 183, 1153–1158 (1992).
Hiratsuka, S., Watanabe, A., Aburatani, H. & Maru, Y. Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nature Cell Biol. 8, 1369–1375 (2006).
Monteiro, A. C. et al. T cells induce pre-metastatic osteolytic disease and help bone metastases establishment in a mouse model of metastatic breast cancer. PLoS ONE 8, e68171 (2013).
Barker, H. E., Cox, T. R. & Erler, J. T. The rationale for targeting the LOX family in cancer. Nature Rev. Cancer 19, 540–552 (2012).
Vora, S. R. et al. Lysyl oxidase propeptide inhibits FGF-2-induced signaling and proliferation of osteoblasts. J. Biol. Chem. 285, 7384–7393 (2010).
Pischon, N. et al. Lysyl oxidase (Lox) gene deficiency affects osteoblastic phenotype. Calcif. Tissue Int. 85, 119–126 (2009).
Feres-Filho, E. J., Choi, Y. J., Han, X., Takala, T. E. & Trackman, P. C. Pre- and post-translational regulation of lysyl oxidase by transforming growth factor-beta 1 in osteoblastic MC3T3–E1 cells. J. Biol. Chem. 270, 30797–30803 (1995).
Bondareva, A. et al. The lysyl oxidase inhibitor, beta-aminopropionitrile, diminishes the metastatic colonization potential of circulating breast cancer cells. PLoS ONE 4, e5620 (2009).
Baker, A. M. et al. The role of lysyl oxidase in SRC-dependent proliferation and metastasis of colorectal cancer. J. Natl Cancer Inst. 103, 407–424 (2011).
Wang, N. et al. Reduced bone turnover in mice lacking the P2Y(13) receptor of ADP. Mol. Endocrinol. 26, 142–152 (2012).
Parfitt, A. M. et al. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J. Bone Miner. Res. 2, 595–610 (1987).
Agrawal, A., Gallagher, J. A. & Gartland, A. Methods in Molecular Biology Vol. 806 (eds Mitry, R. R. & Hughes, R. D. ) 357–375 (Humana, 2012).
Gartland, A., Hipskind, R. A., Gallagher, J. A. & Bowler, W. B. Expression of a P2X7 receptor by a subpopulation of human osteoblasts. J. Bone Miner. Res. 16, 846–856 (2001).
Acknowledgements
We thank the animal welfare staff at the Institute of Cancer Research and Biocentre (University of Copenhagen); the Bone Analysis Laboratory (The University of Sheffield); M. Smid, J. W. M. Martens and J. A. Foekens (Erasmus MC Cancer Institute, Rotterdam, The Netherlands) for in-depth patient data analyses; and A. J. Giaccia and members of our laboratories for comments. This research was supported by funding from Cancer Research UK (C107/A10433) (T.R.C., D.B., G.L.J.T.E.), the Biotech Research and Innovation Centre (BRIC, University of Copenhagen) (T.R.C.), The University of Sheffield (A.G., I.D.H.), National Institute for Health Research Sheffield Clinical Research Facility (A.G.), Breast Cancer Campaign (#2012MayPR086) (A.G., R.M.H.R.), and the Danish Cancer Society (R56-A2971-12-S2) (A.M.H.). Experiments in the laboratory of R.L. were funded by The Lundbeck Foundation and the work was supported by the Velux Foundations (VKR)-funded Instrument Center for Systems Proteomics (VKR 022758). L.P. and J.T.E. are supported by a Hallas Møller Stipendum from the Novo Nordisk Foundation.
Author information
Authors and Affiliations
Contributions
J.T.E. and A.G. conceived the project, assisted by T.R.C. T.R.C., A.G. and J.T.E. designed the experiments. T.R.C., A.G. and R.M.H.R. performed the in vivo and in vitro experiments and analysed the data assisted by L.P., A.H., A.A., D.B., N.A.L., H.F., H.R.E., I.D.H. and G.L. T.R.C. and E.M.S. designed and performed the mass spectrometry and proteomics experiments and analysis, supervised by R.L. T.R.C. wrote and edited the paper, assisted by A.G., J.T.E., R.L. and R.M.H.R.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
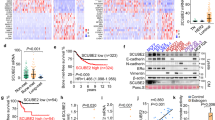
Extended Data Figure 1 LOX is hypoxia regulated and strongly associated with osteotropism and metastasis.
a, Retrospective analysis of our patient cohort including only ER− patients showed that the hypoxic signature is not significantly associated with liver relapse (P = 0.98), brain relapse (P = 0.17) or lung relapse (P = 0.13). b, log2 expression levels under conditions of hypoxia (1% O2) and normoxia (21% O2) for secreted proteins from the MDA-MB-231 parent and MDA-MB-231 bone tropic (BT) 1833 cell line. Data representative of four repeats, two label-free repeats, and two SILAC (standard and reverse-label) repeats. Acquisition performed on the Orbitrap Q-Exactive (Thermo Fisher Scientific). c, Overlaps between repeats of global secretome analysis in MDA-MB-231 parent and MDA-MB-231 bone tropic cells grown in normoxic (21% O2) and hypoxic (1% O2) conditions from label-free and SILAC approaches. d, Immunoblotting for LOX in MDA parent and 1833 bone tropic subclone under conditions of hypoxia (1% O2) and normoxia (21% O2) confirming expression levels seen in proteomic and transcriptomic analyses. Scans of original western blots available as Supplementary Information.
Extended Data Figure 2 Extended patient data analysis.
a, Across all breast cancer patients, the expression of LOX is associated with metastasis formation (P = 0.023) and in particular with ER− breast cancer patients (P = 0.0029). b, An additional Kruskal–Wallis test between reported bone relapse, relapse elsewhere and no relapse patients with an additional contrast test wherein all pairwise groups were considered shows that in all patients LOX expression is associated with bone relapse compared with no relapse (P = 0.0389). This also pertains to ER− patients (P = 0.0126) but not ER+ patients (P = 0.9537). c, Cox-regression using log2(LOX expression data) was used to estimate the hazard ratio in two analyses. One analysis used the no-relapse patients and the bone relapse patients (data belonging to Fig. 1f), and the second analysis included all patients (data belonging to Extended Data Fig. 2a). LOX expression is associated with increased hazard ratio, particularly in ER− patients in both analyses.
Extended Data Figure 3 Additional patient data analysis in a supporting patient cohort.
a, ROC curve analysis shows LOX expression may be indicative of metastatic dissemination of ER− breast cancer (area under the curve 0.77, P < 0.0001) but not ER+ patients (area under the curve 0.55, P < 0.1504). b, In an alternative second patient data set14 (PubMed identifier 12490681) reporting data on 295 lymph-node-negative patients who did not receive adjuvant therapy, with available site of relapse, LOX is significantly higher expressed in bone relapse ER− patients, compared with other groups confirming data from the original data set.
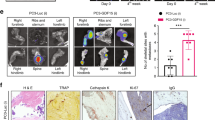
Extended Data Figure 4 Hypoxia-induced tumour-derived LOX stimulates osteolytic lesion formation in the absence of tumour cells.
a, Immunoblotting of 4T1 mammary carcinoma line stably expressing either a scrambled (scr) or shLOX vector which leads to a significant decrease in levels of detectable LOX. Scans of original western blots available in Supplementary Information. b, Micro-CT scanning and reconstruction with structural analysis shows decreases in trabecular bone volume (as a percentage of total bone volume) (n = 3 mice per group). c, Decreases in trabecular number (per millimetre) (n = 3 mice per group) and d, decreases in trabecular thickness in tibiae of mice bearing 4T1scr mammary fatpad tumours over time (n = 3 mice per group). e, Micro-CT analysis of mouse tibiae shows increases in focal osteolytic lesions in 4T1scr tumour-bearing mice develop over time (n = 3 mice per group). *P < 0.05**, P < 0.01, ***P < 0.001, unpaired parametric one-tailed t-test. f, Representative immunohistochemical staining for pimonidazole (Hypoxyprobe) in 4 μm section of 4T1 orthotopic mammary carcinoma, 3 weeks after implantation, shows hypoxia (brown staining) as a salient feature of tumours. Scale bar, 250 μm. g, Bioluminescent imaging of luciferase signal 2 weeks after explant of samples taken from primary tumour, lung, bone marrow (tibia) and distant skin samples at 1–5 weeks after primary tumour implant. Selection was under 500 μg ml−1 zeocin for the luciferase expression cassette (n = 3 mice per time point). h, Quantification of g as a percentage of positive luciferase expressing explants from various sites after 4T1 tumour implant shows tumour cells do not begin to arrive in the bone until 3 weeks. i, qRT–PCR detection of luciferase expressing 4T1 tumour cells in secondary organs confirms explant culture experiments (n = 3 mice per time point).
Extended Data Figure 5 Effects of LOX modulation on circulating sera levels, primary tumour growth and osteolytic lesion formation.
a, ELISA for LOX in the sera of 4T1scr and 4T1shLOX tumour-bearing mice (n: ELISA signal (arbitrary units) in mouse sera: 3 mice per group) shows decreased levels of circulating LOX upon genetic silencing at the primary tumour. *P < 0.05, unpaired parametric two-tailed t-test. b, Growth curves as determined by calliper measurement for orthotopic 4T1scr and 4T1shLOX mammary tumours show no difference between primary tumour growth (n: mice; 3 per group). c, Injection of hypoxic CMs from SW480 human colorectal cancer cells stably expressing one of; empty vector control (EV), full-length LOX (+LOX), or a catalytically inactive full-length LOX (+mutLOX) (K320A) confirms a LOX-dependent mechanism of focal osteolytic lesion generation in a second human model of cancer (n: mice; 8 per group). **P < 0.01, ***P < 0.001, unpaired parametric two-tailed t-test. d, CTX ELISA (RatLaps) on sera of mice injected intraperitoneally twice a week with rLOX for 3 weeks. CTX is a telopeptide that can be used as a biomarker in the serum to measure the rate of bone turnover (n: nanograms per millilitre circulating CTX-I in mouse sera; 5 mice per group). All data are mean ± s.e.m. *P < 0.05, **P < 0.01, ***P < 0.001, unpaired parametric two-tailed t-test.
Extended Data Figure 6 LOX modulates osteoclasts and osteoblast behaviour independently of RANKL.
a, ELISA for RANKL in the CM of osteoclast cultures shows no detectable levels of RANKL in M-CSF alone (negative control) and rLOX cultures excluding the likelihood of autocrine production by cells in response to rLOX (n: ELISA signal (arbitrary units); data are from three independent experimental repeats in all groups). *P < 0.05, unpaired parametric two-tailed t-test. b, All proteins detected by mass spectrometry analysis in the rLOX preparations (based on MaxQuant 1.5 peptide identity score of 50 and a minimum of two unique MS peptide observations). c, Examples of nuclear localization of NFATc1 after addition of rLOX in the presence and absence of the LOX antibody (green, NFATc1; red, phalloidin; blue, DAPI). d, Representative alizarin red S plate showing mineralization ability (calcium deposits as detected by alizarin red S staining) of primary calvarial mouse osteoblasts after treatment with dexamethasone (positive control) or rLOX ± LOX ab; quantification shown in Fig. 3g. e, High-LOX-containing hypoxic 4T1scr CM significantly reduces cell proliferation of the human osteoblast-like SaOS-2 cell line, which can be partly blocked by treatment with anti-LOX antibody (n: normalized cell number per well; control 24 wells; 4T1scr CM 49 wells; 4T1scr CM + LOX Ab 51 wells). Data collected over three independent experimental repeats. *P < 0.05, **P < 0.01, ***P < 0.001, unpaired parametric two-tailed t-test. f, Mineralization ability (calcium deposits as detected by alizarin red S staining) is increased in the human osteoblast-like SaOS-2 cell line in response to high-LOX-expressing hypoxic 4T1scr CM, the effects of which can be attenuated using the anti-LOX antibody (n: alizarin red S staining per well, data taken from three independent repeats; control 18 wells; 4T1scr CM 9 wells; 4T1scr CM + LOX Ab 9 wells). All data are mean ± s.e.m. *P < 0.05, **P < 0.01, ***P < 0.001, unpaired parametric two-tailed t-test.
Extended Data Figure 7 Additional in vivo analysis of lesion formation and primary tumour growth.
a, 4T1scr tumour-bearing mice treated with our LOX antibody show a decrease in osteoclast perimeter in tibial bones in support of LOX as a modulator of osteoclastogenesis shown in Fig. 3 (n: mice; 4T1scr Tumour + IgG 5, 4T1scr Tumour + LOX Ab 7). Data are mean ± s.e.m. *P < 0.05, unpaired parametric two-tailed t-test. b, Weekly tumour volumetric measurements for 4T1scr tumour-bearing mice treated with either zoledronic acid (0.6 mg kg−1 intraperitoneally) or vehicle (PBS), show that, when administered alone, zoledronic acid does not affect primary 4T1scr primary tumour growth in vivo (n: mice; 4 in all groups). c, Pearson correlation shows a positive correlation between lesion number as determined by micro-CT analysis and luciferase signal (radiance (photons per second per square centimetre per steradian)) from 4T1Luc tumour cells within the bone (r = 0.58, 95% CI 0.2778–0.7834, P = 0.0009 (two-tailed)).
Supplementary information
Supplementary Information
This file contains Supplementary Expression data. (PDF 128 kb)
Supplementary Information
This file contains full scans of Western blots. (PDF 502 kb)
About this article
Cite this article
Cox, T., Rumney, R., Schoof, E. et al. RETRACTED ARTICLE: The hypoxic cancer secretome induces pre-metastatic bone lesions through lysyl oxidase. Nature 522, 106–110 (2015). https://doi.org/10.1038/nature14492
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature14492
This article is cited by
-
The Role of Breast Cancer Cells in Bone Metastasis: Suitable Seeds for Nourishing Soil
Current Osteoporosis Reports (2024)
-
Biological characterization of breast cancer spheroid formed by fast fabrication method
In vitro models (2024)
-
Coadaptation fostered by the SLIT2-ROBO1 axis facilitates liver metastasis of pancreatic ductal adenocarcinoma
Nature Communications (2023)
-
Hypoxia induced responses are reflected in the stromal proteome of breast cancer
Nature Communications (2023)
-
The role of integrin family in bone metabolism and tumor bone metastasis
Cell Death Discovery (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.