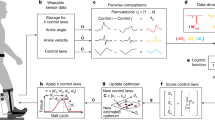
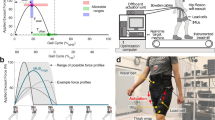
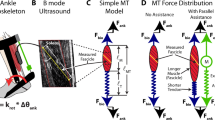
Abstract
With efficiencies derived from evolution, growth and learning, humans are very well-tuned for locomotion1. Metabolic energy used during walking can be partly replaced by power input from an exoskeleton2, but is it possible to reduce metabolic rate without providing an additional energy source? This would require an improvement in the efficiency of the human–machine system as a whole, and would be remarkable given the apparent optimality of human gait. Here we show that the metabolic rate of human walking can be reduced by an unpowered ankle exoskeleton. We built a lightweight elastic device that acts in parallel with the user's calf muscles, off-loading muscle force and thereby reducing the metabolic energy consumed in contractions. The device uses a mechanical clutch to hold a spring as it is stretched and relaxed by ankle movements when the foot is on the ground, helping to fulfil one function of the calf muscles and Achilles tendon. Unlike muscles, however, the clutch sustains force passively. The exoskeleton consumes no chemical or electrical energy and delivers no net positive mechanical work, yet reduces the metabolic cost of walking by 7.2 ± 2.6% for healthy human users under natural conditions, comparable to savings with powered devices. Improving upon walking economy in this way is analogous to altering the structure of the body such that it is more energy-effective at walking. While strong natural pressures have already shaped human locomotion, improvements in efficiency are still possible. Much remains to be learned about this seemingly simple behaviour.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Alexander, R. M. Principles of Animal Locomotion Chs 1 and 7 (Princeton Univ. Press, 2003).
Malcolm, P., Derave, W., Galle, S. & De Clercq, D. A simple exoskeleton that assists plantarflexion can reduce the metabolic cost of human walking. PLoS ONE 8, e56137 (2013).
Lovejoy, C. O. The natural history of human gait and posture: part 1. Spine and pelvis. Gait Posture 21, 95–112 (2005).
Dietz, V. Spinal cord pattern generators for locomotion. Clin. Neurophysiol. 114, 1379–1389 (2003).
Forssberg, H. Ontogeny of human locomotor control I. Infant stepping, supported locomotion and transition to independent locomotion. Exp. Brain Res. 57, 480–493 (1985).
Davidson, P. R. & Wolpert, D. M. Widespread access to predictive models in the motor system: a short review. J. Neural Eng. 2, S313–S319 (2005).
Tudor-Locke, C., Johnson, W. D. & Katzmarzyk, P. T. Accelerometer-determined steps per day in US adults. Med. Sci. Sports Exerc. 41, 1384–1391 (2009).
Ericsson, K. A. & Charness, N. Expert performance: its structure and acquisition. Am. Psychol. 49, 725–747 (1994).
Zarrugh, M. Y., Todd, F. N. & Ralston, H. J. Optimization of energy expenditure during level walking. Eur. J. Appl. Physiol. Occup. Physiol. 33, 293–306 (1974).
Collins, S. H., Adamczyk, P. G. & Kuo, A. D. Dynamic arm swinging in human walking. Proc. R. Soc. B. 276, 3679–3688 (2009).
Westerterp, K. R. Physical activity and physical activity induced energy expenditure in humans: measurement, determinants, and effects. Front. Physiol. 4, 90 (2013).
Yagn, N. Apparatus for facilitating walking, running and jumping. US patent 420, 179 (1890).
Grabowski, A. M. & Herr, H. M. Leg exoskeleton reduces the metabolic cost of human hopping. J. Appl. Physiol. 107, 670–678 (2009).
van Dijk, W., van der Kooij, H. & Hekman, E. A passive exoskeleton with artificial tendons: design and experimental evaluation. Proc. IEEE Int. Conf. Rehabil. Rob. http://dx.doi.org/10.1109/ICORR.2011.5975470 (2011).
Zoss, A. & Kazerooni, H. Design of an electrically actuated lower extremity exoskeleton. Adv. Robot. 20, 967–988 (2006).
Mooney, L. M., Rouse, E. J. & Herr, H. M. Autonomous exoskeleton reduces metabolic cost of human walking. J. Neuroeng. Rehabil. 11, 1–6 (2014).
Rome, L. C., Flynn, L. & Yoo, T. D. Biomechanics: rubber bands reduce the cost of carrying loads. Nature 444, 1023–1024 (2006).
Heglund, N. C., Willems, P. A., Penta, M. & Cavagna, G. A. Energy-saving gait mechanics with head-supported loads. Nature 375, 52–54 (1995).
Zelik, K. E., Huang, T. W. P., Adamczyk, P. G. & Kuo, A. D. The role of series ankle elasticity in bipedal walking. J. Theor. Biol. 346, 75–85 (2014).
Zelik, K. E. & Kuo, A. D. Human walking isn't all hard work: evidence of soft tissue contributions to energy dissipation and return. J. Exp. Biol. 213, 4257–4264 (2010).
Ryschon, T. W., Fowler, M. D., Wysong, R. E., Anthony, A. & Balaban, R. S. Efficiency of human skeletal muscle in vivo: comparison of isometric, concentric, and eccentric muscle action. J. Appl. Physiol. 83, 867–874 (1997).
Grabowski, A., Farley, C. T. & Kram, R. Independent metabolic costs of supporting body weight and accelerating body mass during walking. J. Appl. Physiol. 98, 579–583 (2005).
Roberts, T. J. The integrated function of muscles and tendons during locomotion. Comp. Biochem. Physiol. A 133, 1087–1099 (2002).
Ishikawa, M., Komi, P. V., Grey, M. J., Lepola, V. & Bruggemann, G. P. Muscle-tendon interaction and elastic energy usage in human walking. J. Appl. Physiol. 99, 603–608 (2005).
Bregman, D. J. et al. The effect of ankle foot orthosis stiffness on the energy cost of walking: a simulation study. Clin. Biomech. 26, 955–961 (2011).
Browning, R. C., Modica, J. R., Kram, R. & Goswami, A. The effects of adding mass to the legs on the energetics and biomechanics of walking. Med. Sci. Sports Exerc. 39, 515–525 (2007).
Umberger, B. R. & Rubenson, J. Understanding muscle energetics in locomotion: new modeling and experimental approaches. Exerc. Sport Sci. Rev. 39, 59–67 (2011).
Farris, D. J., Robertson, B. D. & Sawicki, G. S. Elastic ankle exoskeletons reduce soleus muscle force but not work in human hopping. J. Appl. Physiol. 115, 579–585 (2013).
Holt, N. C., Roberts, T. J. & Askew, G. N. The energetic benefits of tendon springs in running: is the reduction of muscle work important? J. Exp. Biol. 217, 4365–4371 (2014).
Kao, P. C., Lewis, C. L. & Ferris, D. P. Invariant ankle moment patterns when walking with and without a robotic ankle exoskeleton. J. Biomech. 43, 203–209 (2010).
Wiggin, M. B., Collins, S. H. & Sawicki, G. S. An exoskeleton using controlled energy storage and release to aid ankle propulsion. Proc. IEEE Int. Conf. Rehabil. Robot. http://dx.doi.org/10.1109/ICORR.2011.5975342 (2011).
Wiggin, M. B., Sawicki, G. S. & Collins, S. H. Apparatus and clutch for using controlled storage and release of mechanical energy to aid locomotion. US patent 2013/0046218 (2013).
Shamaei, K., Sawicki, G. S. & Dollar, A. M. Estimation of quasi-stiffness and propulsive work of the human ankle in the stance phase of walking. PLoS ONE 8, e59935 (2013).
Galle, S., Malcolm, P., Derave, W. & De Clercq, D. Adaptation to walking with an exoskeleton that assists ankle extension. Gait Posture 38, 495–499 (2013).
Winter, D. A. Biomechanics and Motor Control of Human Movement Ch. 7 (John Wiley, 2009).
Farris, D. J. & Sawicki, G. S. Linking the mechanics and energetics of hopping with elastic ankle exoskeletons. J. Appl. Physiol. 113, 1862–1872 (2012).
Sawicki, G. S. & Ferris, D. P. Mechanics and energetics of level walking with powered ankle exoskeletons. J. Exp. Biol. 211, 1402–1413 (2008).
Donelan, J. M., Kram, R. & Kuo, A. D. Simultaneous positive and negative external mechanical work in human walking. J. Biomech. 35, 117–124 (2002).
Brockway, J. M. Derivation of formulae used to calculate energy expenditure in man. Hum. Nutr. Clin. Nutr. 41, 463–471 (1987).
Glantz, S. A. Primer of Biostatistics 65 (McGraw-Hill, 2005).
Tucker, V. A. The energetic cost of moving about: walking and running are extremely inefficient forms of locomotion. Much greater efficiency is achieved by birds, fish—and bicyclists. Am. Sci. 63, 413–419 (1975).
Donelan, J. M. et al. Biomechanical energy harvesting: generating electricity during walking with minimal user effort. Science 319, 807–810 (2008).
Collins, S. H. & Kuo, A. D. Recycling energy to restore impaired ankle function during human walking. PLoS ONE 5, e9307 (2010).
Acknowledgements
We thank A. Westbrook for data collection, K. Takahashi and R. Nuckols for stiffness characterizations, B. Reich for discussions on statistical analysis, R. Jackson for data collection and manuscript editing, and J. Caputo, P. Collins, S. Diller, N. Donahue, A. Kuo, I. Lau, L. Lau, C. Majidi, J. Malen, T. Roberts, A. Robinson, A. Ruina, P. Taggart, K. Witte, M. Wu and others for editorial suggestions. The photograph in Fig. 1b is by S. Thrift. Funding for this research was provided by grants to G.S.S. from the North Carolina State Faculty Research and Professional Development Fund; the North Carolina State Chancellors Innovation Fund; grant number 2011152 from the United States - Israel Binational Science Foundation; and award number R01NR014756 from the National Institute of Nursing Research of the National Institutes of Health. This material is based upon work supported by the National Science Foundation under grant number IIS-1355716 to S.H.C. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or other funding agencies listed.
Author information
Authors and Affiliations
Contributions
G.S.S. and S.H.C. contributed equally to study design and direction; M.B.W., S.H.C. and G.S.S. designed the device; M.B.W. fabricated the device; M.B.W. and G.S.S. conducted human locomotion experiments; M.B.W., S.H.C. and G.S.S. analysed data; S.H.C., M.B.W. and G.S.S. drafted the manuscript; S.H.C. and G.S.S. edited the manuscript. All authors approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare competing financial interests: a non-provisional patent that covers the device has been issued (US2013/0046218 A1, February 2013).
Additional information
Source data are available at https://www.bme.ncsu.edu/labs/hpl/NaturePassiveExoData/.
Extended data figures and tables
Extended Data Figure 1 Energy diagrams for human–exoskeleton walking.
Each diagram includes energy inputs, outputs, storage and transfers within the mechanical system, depicted for steady-state walking. In each case, all chemical or electrical energy input is eventually output as heat, since the mechanical energy of the system is constant on average and no useful work is performed on the body or the environment. Energy efficiency, strictly defined, is therefore zero in all cases, and so energy effectiveness or energy economy is instead characterized in terms of ‘cost of transport’, which is the energy used per unit weight per unit distance travelled41. a, Energy diagram for normal human walking. Muscles consume metabolic energy both to produce mechanical work and to absorb it (and to perform a variety of other functions, such as activating or producing force), and so metabolic energy flows only into the system. Energy loss in muscle manifests as heat. Inside the mechanical system, tendons exchange energy with both the muscle and the body, while kinetic and gravitational potential energy are exchanged within the body segments, all at high mechanical efficiency. Body segment mechanical energy is dissipated only in damping in soft tissues, for example during collisions, which is small (about 3% of the total metabolic energy input20), and in friction from slipping of the feet against the ground, deformation of the ground or air resistance, all of which are negligible under typical conditions. All of these mechanical losses manifest as heat. b, Energy diagram for walking with a powered exoskeleton. An additional energy input is provided in the form of, for example, electricity. The total energy input (and corresponding eventual dissipation) of the system can therefore increase, even if a smaller portion is borne by the human, resulting in poorer overall energy economy. This has been the case with the two powered devices that have reduced the metabolic energy cost of human walking2,16. In theory, overall energy economy could still be improved with a powered device in three ways. First, positive mechanical work from muscles could be replaced by work done by a motor with higher efficiency. Second, negative mechanical work could be replaced by generation done by a motor with higher (than −120%) efficiency, thereby usefully recapturing energy that would otherwise be dissipated as heat. In fact, because muscle expends metabolic energy to absorb mechanical work, it is theoretically possible to simultaneously reduce metabolic rate and capture electrical energy with zero electrical input42, although this has yet to be demonstrated in practice. Third, the powered device could approximate an unpowered device, with negligible amounts of electricity used only to control the timing of mechanical elements such as clutches43. c, Energy diagram for walking with an unpowered exoskeleton. No additional energy supply is provided; so, unlike the powered case, the only way to decrease metabolic energy use is to reduce total system energy dissipation, or, equivalently, to improve the energy economy of the system as a whole. Note that the only difference from normal human walking, in terms of energy flow, is the addition of elements such as springs that store and transfer mechanical energy within the system. In this sense, reducing metabolic rate with a passive exoskeleton is akin to changing the person's morphology such that it is more energy-effective at locomotion.
Extended Data Figure 2 Exoskeleton frame design.
A rigid carbon fibre shank frame and foot frame were custom-made for each participant. The shank section clamps onto the user's lower leg just below the knee and connects to the foot frame through a rotary joint at the ankle. The foot frame includes a lever arm protruding to the rear of the heel, to which the parallel spring is connected. The clutch is mounted to the shank frame posterior to the calf muscles.
Extended Data Figure 3 Ankle moment contributions.
a, Total ankle moment, measured using a motion capture system. Average total ankle moment (b) during the entire stride and (c) during early and mid-stance, defined as 0–40% stride, and (d) peak ankle moment. All spring conditions increased average total joint moment slightly during early stance, but peak total joint moment was maintained across conditions. e, Exoskeleton torque contribution, as measured using onboard sensors. Average exoskeleton torque (f) during the entire stride and (g) during early and mid-stance, defined as 0–40% stride, and (h) peak exoskeleton torque. Average and peak exoskeleton torque increased with increasing exoskeleton spring stiffness, except with the highest stiffness spring. i, Biological contributions to ankle moment, calculated as the subtraction of the exoskeleton moment from the total moment. Average biological ankle moment (j) during the entire stride and (k) during early and mid-stance, defined as 0–40% stride, and (l) peak ankle moment. Ankle moments arising from muscle activity decreased with increasing exoskeleton spring stiffness, but with diminishing returns at high spring stiffness. N = 9; bars, mean; error bars, s.e.m.; P values, two-factor ANOVA (random effect: participant; fixed effect: spring stiffness).
Extended Data Figure 4 Ankle muscle activity.
a, Activity in the soleus, a mono-articular muscle group that acts to plantarflex the ankle. Average soleus activity over (b) the whole stride, (c) early and mid-stance, defined as 0–40% stride, and (d) late stance, defined as 40–60% stride. Soleus activity decreased with increasing spring stiffness. e, Activity in the gastrocnemius, a biarticular muscle group that acts to plantarflex the ankle and flex the knee. Average gastrocnemius activity over (f) the whole stride, (g) early and mid-stance, defined as 0–40% stride, and (h) late stance, defined as 40–60% stride. Gastrocnemius activity was reduced compared with the ‘No Exoskeleton’ condition during early and mid-stance, but increased with increasing spring stiffness during late stance. i, Activity in the tibialis anterior, a mono-articular muscle group that acts to dorsiflex the ankle. Average tibialis anterior activity over (j) the whole stride, (k) early and mid-stance, defined as 0–40% stride, and (l) late stance, defined as 40–60% stride. Tibialis anterior activity seemed to increase during early and mid-stance, and was unchanged during late stance. All values were measured using electromyography and normalized to maximum activity during normal walking. N = 8; bars, mean; error bars, s.e.m.; P values, two-factor ANOVA (random effect: participant; fixed effect: spring stiffness).
Extended Data Figure 5 Ankle power contributions.
a, Mechanical power of the combined human–exoskeleton system, measured using a motion capture system, (b) average positive power, defined as positive work divided by stride time, (c) average negative power, defined as negative work divided by stride time, and (d) average net power, equivalent to average power, defined as the sum of positive and negative work divided by stride time. Total positive ankle joint power decreased with increasing stiffness, while net joint power increased. e, Exoskeleton power, measured using onboard sensors for torque and motion capture for joint velocity, (f) average positive exoskeleton power, (g) average negative exoskeleton power and (h) average net exoskeleton power. Net exoskeleton power was always negative. i, Biological ankle power, defined as the subtraction of exoskeleton power from total ankle power, (j) average positive biological power, (k) average negative biological power and (l) average net biological power. Net biological power increased with the exoskeleton compared with normal walking. N = 9; bars, mean; error bars, s.e.m.; P values, two-factor ANOVA (random effect: participant; fixed effect: spring stiffness).
Extended Data Figure 6 Knee moment.
a, Knee moment in time as measured by motion capture, (b) average absolute knee moment over the entire stride, (c) average knee moment during early stance, defined as the positive impulse within approximately 10–30% stride divided by stride period, and (d) average knee moment during late stance, defined as the negative impulse within approximately 30–50% stride divided by stride period. Average knee moment during late stance increased in magnitude with the highest stiffness springs. Positive values denote knee extension. N = 9; bars, mean; error bars, s.e.m.; P values, two-factor ANOVA (random effect: participant; fixed effect: spring stiffness).
Extended Data Figure 7 Hip, knee and ankle joint mechanics.
Joint angles, moments and powers are presented at the same scale to facilitate comparisons across joints. a, Hip joint angle, (b) knee joint angle and (c) ankle joint angle. Joint angle trajectories did not appear to change substantially across conditions. d, Hip moment, (e) knee moment and (f) biological component of ankle moment. Hip moment did not appear to change substantially across conditions, while knee moment and ankle moment showed trends detailed in Extended Data Figs 6 and 3, respectively. g, Hip joint power, (h) knee joint power and (i) the biological component of ankle joint power. Hip and knee power did not appear to change substantially across conditions, while biological ankle power showed trends detailed in Extended Data Fig. 5. Positive values denote hip extension, knee extension and ankle plantarflexion with respect to standing posture. N = 9.
Extended Data Figure 8 Centre-of-mass mechanics.
a, The biological contribution to centre-of-mass power for each individual limb, defined as the dot product of ground reaction force with centre-of-mass velocity, both determined from force plate data, minus the ankle exoskeleton power. b, Average collision power, defined as the negative work performed during the first half of stance divided by stride time. c, Average rebound power, defined as the positive work performed during mid-stance divided by stride time. d, Average preload power, defined as the negative work performed during mid-stance divided by stride time. e, Average push-off power, defined as the positive work performed during late stance divided by stride time. With increasing spring stiffness, the human contribution to push-off work decreased, while the human contribution to rebound work increased substantially. N = 9; thin lines, contralateral limb; bars, mean; error bars, s.e.m.; P values, two-factor ANOVA (random effect: participant; fixed effect: spring stiffness).
Supplementary information
Supplementary Information
This file contains Supplementary Methods, a Supplementary Discussion and additional references. It includes a detailed description of the custom mechanical clutch; a description of the ‘normal barrier’ of energy cost of walking and a survey of previous attempts to break it; and detailed interpretations of the physiological reasons for the observed reduction in metabolic rate. (PDF 438 kb)
Supplementary Table 1
This file contains all results for both primary and secondary outcome measures and ANOVA statistics for these measures. (XLSX 37 kb)
Supplementary Data 1
This file contains a list of materials and mechanical drawings of the unpowered clutch design. (PDF 1525 kb)
Supplementary Data 2
This zip file contains CAD files depicting the passive clutch design in detail, suitable for reproduction of the system. (ZIP 6361 kb)
Demonstration of the exoskeleton’s function during walking
A demonstration of the exoskeleton’s function during walking, with events annotated, played back at normal and reduced frame rates. (MP4 27802 kb)
Video description of the clutch
A video description of the clutch, with stages of operation described. (MP4 18784 kb)
Rights and permissions
About this article
Cite this article
Collins, S., Wiggin, M. & Sawicki, G. Reducing the energy cost of human walking using an unpowered exoskeleton. Nature 522, 212–215 (2015). https://doi.org/10.1038/nature14288
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature14288
This article is cited by
-
NSF DARE—transforming modeling in neurorehabilitation: a patient-in-the-loop framework
Journal of NeuroEngineering and Rehabilitation (2024)
-
Quantifying changes in individual-specific template-based representations of center-of-mass dynamics during walking with ankle exoskeletons using Hybrid-SINDy
Scientific Reports (2024)
-
Review of Power-Assisted Lower Limb Exoskeleton Robot
Journal of Shanghai Jiaotong University (Science) (2024)
-
Comparison of five different methodologies for evaluating ankle–foot orthosis stiffness
Journal of NeuroEngineering and Rehabilitation (2023)
-
The interaction between muscle pathophysiology, body mass, walking speed and ankle foot orthosis stiffness on walking energy cost: a predictive simulation study
Journal of NeuroEngineering and Rehabilitation (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.