At GlaxoSmithKline, we never lose sight of the fact that patients are at the core of what we do and that millions of lives are impacted by cancer. The patient’s voice is at the heart of our company, and the people for whom these medicines are designed are a vital part of our research and development (Fig. 1). The insights they have provided through their partnership in pan-oncology and disease-specific patient councils are reflected in the design of clinical trials and in building GSK’s pipeline of novel oncology programmes to meet their needs.
As part of our commitment to delivering transformational medicines to patients, we are pioneering innovation and evolution in trial design to complement the development of novel therapies. These cutting-edge approaches, such as adaptive studies, umbrella protocols and platform trial designs, have been used infrequently in oncology and other therapy areas to date but have the potential to expand and accelerate clinical development capabilities beyond a traditional research framework. In particular, a platform trial design allows efficient evaluation of a drug in combination with agents with different mechanisms of action, with the goal of achieving synergistic effects and further enhancing anti-cancer activity without compromising safety. Novel combinations with multimodal mechanisms of action are particularly important in blood cancers, in which a significant amount of cytogenetic variability exists, and genetic plasticity over time enables the cancer cell to evade the immune system and develop resistance to treatments1,2. GSK oncology is piloting the platform clinical trial approach because it allows for investigational agents from the four pillars across our oncology pipeline (immuno-oncology, oncology cell therapy, cancer epigenetics and synthetic lethality) to be combined and efficiently evaluated. It is our hope that such an approach will progress drug development to answer the substantive unmet need for effective therapies for patients with haematologic malignancies and other cancers.

Figure 1. People are at the core of all that we do at GlaxoSmithKline.
Treatment challenges in haematologic malignancies
Haematologic cancers, comprising lymphoid and myeloid malignancies (derived from lymphocytes or other blood cells, respectively), are a key area of research and development interest for GSK3. Cancers of the blood and lymphatic system (lymphomas, leukaemias and myelomas) account for around 7% of newly diagnosed cancers globally, with non-Hodgkin lymphoma being the most commonly diagnosed among this group4. Haematologic malignancies present unique challenges for treatment3,5. Their systemic nature and intricate involvement of the immune system make them more difficult to treat than localized solid tissue cancers5. Primary and acquired drug resistance are common, complicated by the phenomenon of chromosomal translocation, a mechanism of genetic change more typical in haematologic than solid malignancies that creates an evolving genetic environment3,5. Indeed, a particular challenge in haematologic oncology lies in the prevention of relapse and the development of a relapsed and refractory form of the disease that brings dramatically reduced life expectancy3,5. In addition, chemotherapy for haematologic malignancies tends to be more intensive than for solid tissue cancers, and there is a higher requirement for hospital-based treatments, which triggers concerns for tolerability and impact on quality of life, especially in older patients6. There is a pressing need for new treatments for patients with haematologic malignancies that can address these challenges.
In multiple myeloma specifically, despite recent advances in management that have improved overall survival, not all patients receive clinical benefit from available therapies, and disease relapse is common7. Unravelling the biology of relapsed or refractory multiple myeloma presents a notable hurdle due to its heterogeneous nature and the diverse treatment regimens applied in this setting1,7. Furthermore, because prognosis is poor in relapsed or refractory multiple myeloma, efficient selection of the right drug or drug combination for each patient is critical to maximize the chances of treatment success. Optimizing the treatment paradigm on a per-patient basis is a significant challenge in multiple myeloma treatment. Due to enduring unmet patient needs, multiple myeloma is a field of significant research interest, as evidenced by the large number of clinical trials ongoing in multiple myeloma. These trials are examining the utility of agents targeting a range of pathways and receptors, including programmed cell death protein-1 (PD-1), cluster of differentiation (CD)38, B-cell maturation antigen (BCMA), OX40 and inducible T-cell co-stimulator (ICOS), to name a few. Combinations of novel and more established agents are also being examined, in hopes of increasing anti-cancer activity without compromising patient safety. Multiple myeloma is therefore an area in which great change is expected. GSK oncology is committed to facilitating this change through the discovery and development of new oncology compounds and combinations that leverage patient-driven science to deliver improved outcomes for more patients.
Novel oncology drug combinations
One way in which GSK hopes to facilitate change in evolving treatment landscapes, particularly in haematologic malignancies, is through the use of platform clinical trials (Fig. 2). Platform trials are designed to study multiple targeted therapies in the context of a single disease in a perpetual manner with sub-studies running concurrently; therapies can be added or removed from the platform based on the results from a prespecified algorithm8. The advantage of platform trials is that they allow direct comparisons between different therapies/combinations or can be structured to evaluate different treatments/combinations compared with their respective controls in parallel8,9. At GSK, we utilize a master protocol to allow evaluation of multiple novel combinations against a shared monotherapy control arm.
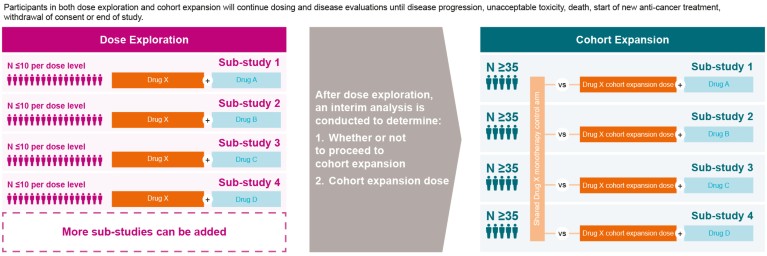
Figure 2. Example GlaxoSmithKline oncology platform clinical trial.
Sub-studies within the master protocol include combinations of agents with different mechanisms of action that are selected based on scientific rationale and/or results from preclinical experiments in combination with the agent in the monotherapy control arm. Our trials consist of a dose exploration phase of each sub-study to evaluate the safety, tolerability and clinical activity of escalating doses and to identify a recommended dose for cohort expansion for each investigational combination treatment. Starting doses for each investigational agent in a combination are selected based on findings from previous monotherapy studies. Sub-studies may additionally involve dose escalation or de-escalation cohort(s), guided by a modified toxicity probability interval method10. The objectives of each of the sub-studies in the platform trial are similar. In the dose exploration phase, the primary objectives in each sub-study are to determine the safety and tolerability of the combination and to establish the dose for cohort expansion for the combination treatment. Key secondary objectives include evaluation of clinical measures of efficacy for each of the novel combinations.
After the dose exploration phase of a sub-study is completed, an interim analysis is conducted to determine whether or not to proceed to a cohort expansion phase and to determine the cohort expansion dose. The primary objective of the cohort expansion phase is to assess the efficacy of the combination at the selected dose versus the monotherapy control arm; secondary objectives include further assessments of the clinical activity and safety of the combination. Efficacy, safety and therefore the feasibility of continuing the line of enquiry are determined early in the process, and additional sub-studies and controls can be added at later stages of the study, if needed. GSK is utilizing the platform clinical trial approach in haematologic malignancies (relapsed or refractory multiple myeloma in particular) with the hope of efficiently identifying novel combinations that will address the substantial unmet medical need in this patient population. The investigational agents being tested in such a platform trial are drawn from the extensive GSK oncology pipeline (as detailed below), as well as from collaborations with external partners.
Challenging questions in cancer research
At GSK oncology we have prioritized our research efforts into four key areas that we believe offer the greatest potential for transformational medicines that can help patients diagnosed with cancer (Fig. 3). Investigational agents within some of these research areas are already being assessed in combination using the platform clinical trial approach.
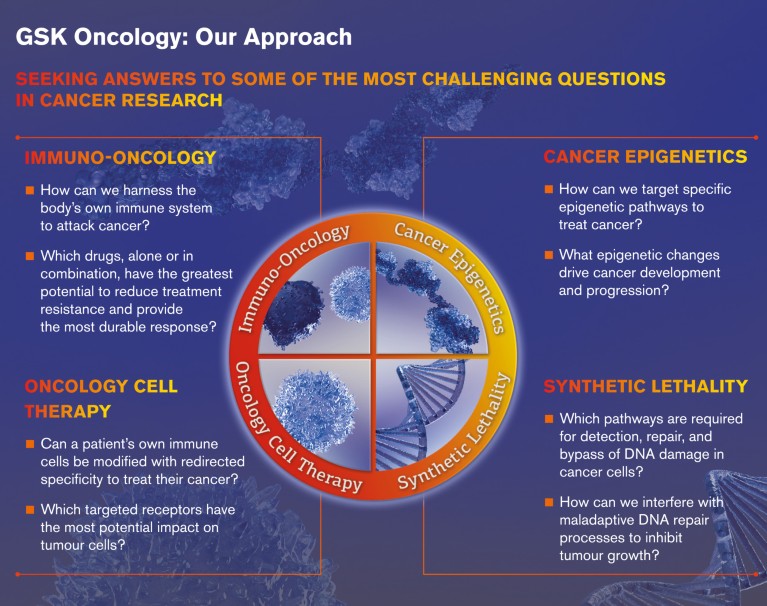
Figure 3. GlaxoSmithKline drives cancer drug discovery through patient-driven science and trailblazing research.
Immuno-oncology
Stem cell transplantation heralded the era of ‘targeted therapy’ in haematologic malignancies. Indeed, the curative potential of this therapy sparked an appreciation of the power of our own immune systems in the battle against blood cancers5. The growing understanding of tumour cells’ ability to evade immune surveillance has led to advances in the field of immuno-oncology (Fig. 4). Malignant cells manipulate a variety of physiological mechanisms involved in antigenicity, immune activation, T-cell priming and recruitment and upregulation of checkpoint molecules11. Many of these mechanisms may be impacted simultaneously to promote tumour cell survival. Immunotherapies harness the body’s own immune system to fight cancer by using different immunological pathways to enhance anti-tumour responses11,12.
GSK is exploring different clinical assets aimed at augmenting the immune response, reducing immune suppression and modulating the tumour microenvironment. Haematologic malignancies are an ideal fit for immune-directed intervention due to their close involvement with sites of immune origin, immune responsiveness and the ease of isolation and manipulation of cancer cells13. Research in this therapeutic area is being applied to try to unravel the complex genetic web behind each type and sub-type of haematologic cancer. Indeed, several assets within our immuno-oncology pipeline are being explored as novel combinations for the potential treatment of relapsed or refractory multiple myeloma using the platform clinical trial model.
Figure 4: Patient T cell (red) targeting malignant plasma (multiple myeloma) cell (blue).
Oncology cell therapy
The physiologic role of central and peripheral tolerance mechanisms is to limit unchecked immune responses that can lead to autoimmunity. In cancer, these mechanisms are major limitations to effective T-cell mediated anti-tumour immunity14. Oncology cell therapy uses adoptive transfer of engineered T cells that may mediate anti-tumour effects. In adoptive cell therapy, T cells are isolated from the patient, engineered to present an enhanced T-cell receptor that recognizes a specific antigen and then reintroduced into the patient14,15. This innovative approach generates T cells that may be more efficient at targeting cancer cells and may overcome the barriers of tolerance mechanisms15. GSK is developing an engineered T-cell receptor, T-cell immunotherapy designed to recognize a tumour-specific antigen and eliminate malignant cells in solid tumours and haematologic malignancies.
Cancer epigenetics
Aberrant gene expression, regulated in large part by epigenetic mechanisms, is a hallmark of cancer16. ‘Epigenetics’ refers to heritable changes in gene expression that arise from changes in chromosomes without altering the DNA sequence17. DNA methylation and post-translational modifications of histones play key roles in regulating gene expression16. Deregulation of these epigenetic mechanisms can lead to aberrant expression of oncogenes and tumour suppressors in cancer cells that can enhance proliferative signals, impair cell death, promote angiogenesis and facilitate metastasis17. GSK is investigating compounds that work by altering these epigenetic pathways in both solid tumours and blood cancers.
Synthetic lethality
Accumulation of DNA damage and genomic instability are pervasive characteristics of human tumours and are caused by defects in DNA repair18,19. Deficiencies in essential DNA damage repair in cancer cells may increase dependency on an alternate repair pathway for cell survival19. Synthetically lethal therapies aim to combine pharmacologic inhibition of these alternate repair pathways with inherent defects in DNA damage repair to selectively kill tumour cells while sparing healthy tissue19,20. While the importance of synthetic lethality in the potential treatment of haematologic malignancies is not clear, GSK is committed to investigating this approach using a variety of solid tumour cancer models.
Summary
GSK’s research and development foundation, ‘Science x Technology x Culture’, is driving the innovation (science) x investment (technology) x patient centricity (culture) behind our approach to maximize survival in patients with haematologic and other malignancies. We believe that our flexible and innovative approach to trial design, together with our robust investigational pipeline, will drive towards transformative single-agent and combination medicines for the treatment of haematologic and other cancers.

