As scientists, our focus is on creating a different future. Because we are engaged in the discovery and development of new medicines, we have the extraordinary responsibility to prolong and enhance the lives of other humans. We do that by better understanding how cells perform their functions, how healthy homeostasis is maintained and what causes a disease phenotype. We then use that information, coupled to drug discovery technologies, to create medicines that have acceptable therapeutic indexes or risk–benefit ratios. Thus, as scientists, we think of history as the prologue to the future.
Traditional drug-discovery efforts focus on designing small molecule agents (usually <500 Dalton) meant to bind to and alter the structure and function of specific proteins. It has been apparent for many decades that this process is inadequate to meet the needs of patients or the demands of industry for new products. Moreover, these inefficient drug discovery systems often fail to take advantage of the extraordinary advances in understanding the molecular mechanisms of disease. There are numerous causes for the decline in productivity of our industry. For example, although the development of antimicrobial resistance is an issue, in the developed world infectious diseases are well managed, as are many acute illnesses. This means that the industry is, to a large extent, focused on improving the therapy of chronic degenerative diseases. The treatment of chronic diseases is intrinsically a more difficult challenge for drug discoverers and developers. Increased demands from regulators before approval of new medicines, particularly the demand for high risk, enormously costly outcome studies, are a significant determinant. The evolution of monoclonal antibodies is representative of new technology that is more efficient and takes better advantage of the advances in understanding the molecular pathology of diseases that small molecule drugs are rarely able to address. But more must be done.
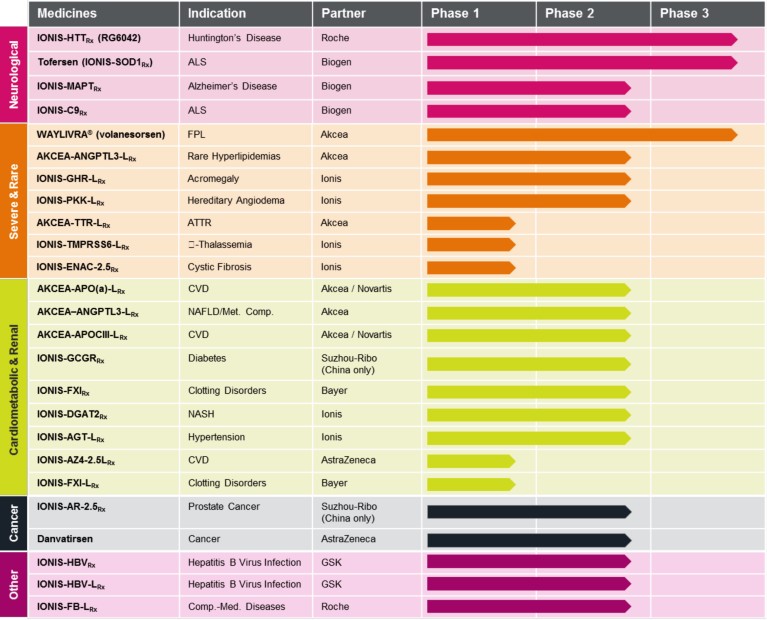
Figure 1. Drugs in development. Current pipeline at Ionis adapted from the annual meeting presentation June 6, 2019.
An entirely new chemical class of medicines
Thirty years ago, armed with a blank piece of paper and US$5.2 million, Ionis set out to create a radically different drug discovery technology. We planned to design an entirely new chemical class of medicines called chemically modified oligonucleotides that would bind to their targets through an interaction never before used for drug discovery: Watson–Crick hybridization. We also planned to focus on molecules that had never been considered as drug receptors: ribonucleic acids (RNAs). We were not alone. Gilead, Hybridon and Genta were founded before or contemporaneously with Ionis, but we stayed the course. Because of 30 years of progress, we believe that we can realize an extraordinarily exciting future. The next few pages introduce the future envisaged by Ionis.
At Ionis, we have developed and achieved regulatory approval for five first-in-class medicines, including the first RNA-targeted blockbuster, Spinraza. Currently, we have more than 40 first- or best-in-class medicines in development and expect at least 10 or more drugs to be in pivotal trials or registration1 by the end of 2020. Because of the efficiency of RNA-targeted drug discovery as practiced at Ionis and our unique business model, we produce one medicine for every 12 Ionis employees (we refer to ourselves as Ions). That represents a large and growing competitive advantage compared to the 1,000 plus persons required for the fully integrated companies using other available drug discovery platforms. Today, we have fully validated intravenous (IV), subcutaneous (SC) and intrathecal (IT) administration. We also have encouraging data on aerosol administration and can give antisense oligonucleotides (ASOs) as enemas (Fig. 1). Over the next several years, we intend to grow our pipeline to approximately 60 medicines, with 10 being in pivotal trials at steady state. Because of the efficiency of the platform we can enlarge our pipeline with very modest increases in our staff. This support assures the core of Ionis never exceeds 500 or so people. Why 500? Because organizational studies have shown that about 500 is the optimal size to assure maximal innovation.
Innovation that brings maximum value to patients
In our business, the number of medicines in development matters a great deal because they provide more opportunities for success. However, our focus goes well beyond numbers. We are intent upon pursuing novel molecular targets that have the potential to revolutionize the therapeutic landscape and patient lives. Given the versatility, breadth of utility and the success rate of medicines derived from our platform, we can afford to take greater target risk. It is only by taking such an approach that transformative first-in-class medicines can be developed. We are committed to bringing the maximum value to the patients who need it the most because we feel that should be the mandate of our industry. And because we strongly believe that providing value to patients and charging for the value provided is the future of our industry. ASOs are a direct route from the gene to the patient and we fully expect that genomic advances will be continually incorporated in all our drug discovery and development activities.
In our pipeline of 2025, we expect to have fully enabled aerosol delivery of ASOs for respiratory diseases such as chronic bronchitis, commercially attractive, orally administered medicines and new chemical approaches that provide potent agents to treat diseases of the heart and skeletal muscles. Because ASOs that are members of the same chemical class have very similar properties (differing only in their sequence), we pioneered informative systems that integrate all safety data from studies in non-human primates and all randomized, placebo-controlled trials and have published the results of those analyses2,3,4,5,6. As new chemistries and routes of administration evolve, we will assemble and publish the results. These databases are of extraordinary value because learnings from previously developed medicines help inform our expectations to newer medicines in a particular chemical class. This contributes to the much higher success rate we see compared to more traditional approaches.
Although we are committed to bringing new therapies like Spinraza, Tegsedi and Waylivra to patients with rare diseases, our platform is also capable of producing medicines to treat more common disorders. Today, about 30% of our pipeline is committed to the treatment of rare diseases. By 2025 we expect the pipeline to be about 20% rare disease and 80% more common diseases. Importantly, we expect the performance of our medicines to continue to improve. The improved performance will be driven by advances made by the Ionis core antisense program. Today, we have medicines that can be administered subcutaneously at 10-20 mg per month or less frequently. We expect the potency of our agents to continue to increase, enabling even more cost effective and better tolerated agents. The increase in potency should support the treatment of new types of diseases such as cardiac arrhythmias and congestive heart failure.
On the cusp of ‘designer’ medicines
Certainly, an exciting future, but the most exciting opportunities are enabled by very recent advances in understanding the molecular mechanisms by which our medicines distribute in vivo and within cells and the molecular mechanisms by which they produce both pharmacodynamic and toxic effects. By exploiting this new knowledge, we believe we are on the cusp of achieving the dream of designer medicines. For the first 25 years, we focused our medicinal chemistry efforts on enhancing the interactions of ASOs with targeted RNAs. That effort has yielded what we call second generation and generation 2.5 ASOs that are substantially more potent, can be administered much less frequently by essentially all routes and are much safer than first generation ASOs. Given that we literally began with a blank piece of paper, this effort has been extraordinarily successful and resulted in our dominant intellectual position in RNA targeted therapeutics.
About five years ago, based on important new data and understanding, our focus in research to advance the performance of ASOs expanded to include attempting to improve the interactions of ASOs with target RNAs. Two simple sounding statements describe the insights that have profoundly influenced our research and the future we think is possible for the technology. The fate of phosphorothioate (PS) ASOs is defined by the proteins with which they interact. PS ASOs can significantly alter the fate of many proteins with which they interact. These observations, combined with the knowledge that as much as 99% of a dose of an ASO is not localized at the desired sites for maximal activity, resulted in the establishment of four new research directions: (i) ligand conjugated ASOs or LICA tissue targeting; (ii) the targeted intracellular distribution; (iii) the design of ASOs to take advantage of newly identified molecular mechanisms of action and; (iv) the molecular toxicology program.
Now that we have substantial knowledge about the key proteins with which ASOs interact in plasma and other in vivo sites, we have established an effort to conjugate ligands (LICA) to ASOs to direct a greater fraction of a dose to desired tissues and cells. The initial and, to date, most important success was achieved first at Alnylam7 and then quickly adopted by Ionis. By conjugating N-acetylgalactosamine (GalNAc) to an ASO, it is possible to take advantage of a high capacity receptor on the surface of hepatocytes, the asialoglycoprotein receptor, to enhance productive delivery of ASOs to hepatocytes. This has consistently increased the potency of second generation ASOs for hepatic targets by approximately 30-fold. When conjugated to the generation 2.5 ASOs, which are about 10 times as potent as generation two drugs, this ligand increased potency again by about 30-fold. Based on this success, we are now concentrating efforts on identifying ligands that enhance delivery of ASOs to other tissues and cells. In collaboration with our colleagues at Astra-Zeneca we recently reported that by attaching a GLP–1 peptide to an ASO we can achieve therapeutic ASO concentrations in the beta cells of the pancreas8. As this ligand is optimized, we expect to create medicines that enhance the metabolic control effected by these cells. We have also made significant progress in identifying ligands that enhance delivery of ASOs to the heart, skeletal muscle and other cells. Although more work is required, we are optimistic that we will enhance the performance of ASOs in many tissues that intrinsically take up lower fractions of a dose of an ASO.
Similarly, cellular uptake and distribution of ASOs is very inefficient. Based on recent work performed primarily in our laboratory, we now understand the major pathways by which cells take up and distribute PS ASOs, the major proteins that are involved and the main sites in the cell in which ASOs are active. We also have a solid understanding of the kinetics of these processes (Fig. 2). Importantly, we have identified straightforward medicinal chemical modifications that can substantially alter the interactions of PS ASOs with the proteins responsible for the transport of ASOs across the plasma membrane and those that distribute ASOs inside the cell. We know that approximately 99% percent of the ASOs in the cell are in locations in which the ASO is not active, so a shift of just 1% from an inactive intracellular site to an active site should double potency9.
The third core research theme that we expect to have a significant impact on the future of the technology is actually not a new effort. We have worked for 30 years to understand the major steps involved in the molecular mechanisms of pharmacodynamics, the kinetics of these steps and the factors that influence the effectiveness of the desired pharmacology of ASOs. We have achieved most of those goals10. Recent progress in this area is yielding data that potentially enhance the future value of the platform. For example, we now understand one of the major mechanisms of tolerance to ASOs and this process also likely explains why some transcripts are less effectively reduced by ASOs designed to activate RNAse H1. Perhaps, more importantly, we have expanded the repertoire of mechanisms for which we can design ASOs including those that, for the first time, selectively increase the translation of specific proteins, thus serving as ‘agonist -like drugs’11.
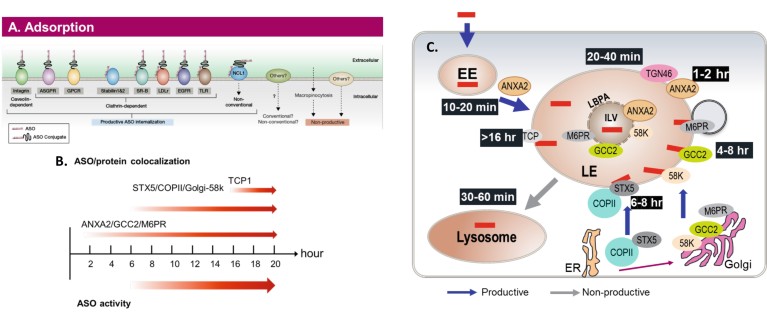
Figure 2. The uptake and distribution pathways and correlation with ASO activity8. (A) Cell surface interactions. The proteins located on the cell surface that are known to interact with PSASOs and/or ligands conjugated to ASOs. The interactions with proteins that are known to enter pathways that lead to observable ASO activity in cells (productive pathways) and those that appear to result in cell uptake, but no ASO activity (non-productive) are shown. (B) The timeline of PSASO localization in key endosomal structures and with key proteins vs the ASO activity. (C) Detailed time course of PSASO traffi cking in productive endosomal pathways. Adapted from (9).
Opportunities to design even better ASOs
The fourth new research direction is arguably the most important because it resolves one of the most controversial topics in the technology and provides straightforward medicinal chemical solutions to the issue. The problem is that as ASOs are screened, we occasionally encounter some compounds that are toxic. The toxicities are influenced by the sequence of the ASO and the type of 2'chemical modifications employed. We outlined a step-by-step molecular mechanism that accounts for these observations (Fig. 2) and a straightforward medicinal chemical approach that ablates or substantially reduces toxicities with minimal effects on potency10. We have expanded the database supporting this mechanism and greatly expanded the structure activity and sites of placement of modifications that ablate or reduce toxicities and enriched our understanding of the mechanism. These observations have further enhanced the efficiency of already very efficient screening methods, enhanced the overall potency of agents for development and opened new areas for medicinal chemistry.
Considering the impact of the four research themes enumerated, it is clear that we are close to realizing the dream of designer medicines: therapeutic agents that distribute in the body to perform the jobs for which they are designed, localize intracellularly to be more potent and less likely to have undesired effects, meeting a wide range of pharmacologic needs through a variety of pharmacodynamic mechanisms, and finally, avoiding the possibility of side effects other than those that might be associated with the molecular target. These are realistic opportunities that are the product of knowledge accumulated at Ionis through 30 years of persistent and unprecedented efforts in the history our industry. That, to Ionis, is an extraordinary future.

