At Sanofi Pasteur, we believe in a world in which no one suffers or dies from a vaccine-preventable disease. As the world’s leading manufacturer of influenza vaccines, we know influenza is still one of the most devastating, yet under-appreciated, diseases of modern society, despite the availability of effective vaccines. According to the World Health Organization (WHO), seasonal influenza epidemics are estimated to result in 3 to 5 million cases of severe illness worldwide and 290,000 to 650,000 deaths annually1. Among 31 human infectious diseases, influenza topped the list in burden of disease and disability-adjusted life years (DALYs)2. Because of this, work is urgently needed to reduce the effects of influenza on human suffering and death. We believe this is achievable by increasing the awareness of the serious and far-reaching impact influenza has on human health; by improving the understanding of the benefits of today’s influenza vaccines; and by leading research and development efforts to provide new, more effective influenza vaccine options.
Influenza remains one of the most devastating diseases of modern society.©iStock
Influenza: more than an acute respiratory infection
For more than 60 years, manufacturers have been tackling influenza infection and its significant consequences with effective vaccines. Studies describing the safety, efficacy and effectiveness of influenza vaccines unequivocally demonstrate the favourable benefit/risk ratio of immunization for those at high risk, such as the elderly or immunocompromised individuals. However, society still negates the seriousness of influenza by often considering it no worse than a bad cold. Despite clear recommendations from national and international health authorities, vaccine coverage rates among eligible patients in many countries remain below 50%. There are several reasons for this, top among which are misinformation and misperceptions of the personal risk or the potential serious outcomes of influenza infection. Sanofi Pasteur aims to increase the awareness of influenza as a severe disease, to ensure that the maximum public-health impact of current vaccines is achieved, while we continue research to develop new and more effective vaccines.
While the public-health burden associated with annual influenza epidemics and the positive impact of vaccines are well documented, communication efforts tend to focus on laboratory-confirmed cases. This is a diagnosis infrequently established in the day-to-day clinical setting, and one that hides a multitude of different clinical courses and outcomes. It is true that influenza starts with a sudden onset of symptoms associated with respiratory infection, and recovery can occur within a few days to weeks without further consequences. What many do not understand is that life-threatening complications can develop, even in the young and otherwise healthy.
An influenza infection can both trigger acute health conditions, such as pneumonia, myocardial infarction and stroke, and exacerbate chronic conditions, like diabetes and respiratory illness. However, influenza and its health complications are underdiagnosed. If more people were aware of these complications, and thus the value of vaccination, they would be more convinced to get vaccinated.
For almost 100 years, an association between influenza and cardiovascular disease has been recognized due to overlap in their peak incidence during winter months. Epidemiological studies have also described an increase in cardiovascular deaths during influenza epidemics. Taken together, these observations suggest cardiovascular complications of influenza infection can be significant. Vaccine studies have provided further support that influenza vaccination can help prevent or reduce the risk of many cardiovascular complications3,4. In fact, estimates of influenza vaccine effectiveness to help prevent acute myocardial infarction range from 15% to 45%, comparing favourably with other more routine coronary prevention measures, including smoking cessation (32–43%), statins (19–30%) and antihypertensive therapy (17–25%)5.
Those with chronic lung disease, such as chronic obstructive pulmonary disease (COPD), often find their medical conditions worsen as a result of influenza infection. Influenza vaccination has been shown to be associated with reduced hospitalizations among people with diabetes, chronic lung disease and cardiovascular disease. Therefore, annual vaccination should be an obligatory component of chronic disease-management programmes.
If we are to ensure every patient receives an influenza vaccine whenever advocated, stakeholders must be convinced that an infection can not only be serious in its own right, but can also lead to acute health crises such as heart attack or stroke, and/or exacerbate underlying chronic conditions like diabetes or respiratory illnesses.
Determinants of vaccine effectiveness
As evidenced, vaccination remains the cornerstone of preventing influenza infection and related complications. However, the overall vaccine effectiveness against influenza still varies considerably year over year. Over the last 15 years in the USA, estimates of vaccine effectiveness to prevent medically attended laboratory-confirmed influenza have varied between 10% and 60%6. Yet, even with modest vaccine effectiveness, the impact of annual vaccination campaigns is profound and should not be ignored. Following the 2017–2018 season, the US Centers for Disease Control and Prevention (CDC) reported influenza vaccine effectiveness of only 38%, which meant that vaccination still prevented an estimated 7.1 million illnesses, 3.7 million medical visits, 109,000 hospitalizations and 8,000 deaths7. To that end, efforts in influenza prevention should not lose focus on the benefits of today’s vaccines, several of which have improved effectiveness compared to traditional influenza vaccines. The use of current vaccines is critical, while at the same time we continue to look for ways to develop even more effective, broadly protective vaccines.
To understand how to improve influenza vaccines we must take into account the variety of factors that result in suboptimal and variable effectiveness including antigenic drift of circulating viruses, immune waning following vaccination and weakened immune responses due to a number of potential human host factors.
Antigenic drift
The first of these, antigenic drift, occurs when the circulating influenza virus mutates and is no longer recognized by the antibodies induced by the vaccine. Current vaccines do not have a sufficient ability to induce broad cross-protection against drifted or shifted circulating strains; their effectiveness is driven by how well the strains in the vaccine are matched with the circulating strains. This is why, since 1952, the WHO and country regulatory authorities maintain an extensive influenza-surveillance system and select the recommended composition of vaccines for each season. Maintaining such an effort is necessary, but difficult and time-consuming. Despite best efforts and up-to-date information, the strains selected for vaccine production may not adequately match those circulating during the following influenza season, resulting in mismatch. New vaccines, including those being developed by Sanofi Pasteur, to induce an immune response that is not negatively impacted by viral antigenic drift would be a tremendous advance.
Immune waning
Second, multiple reports from Europe, Australia and the USA report that vaccine effectiveness wanes during the influenza season at varying degrees, and depends on the vaccine strain and, potentially, host factors. While the effect of waning may be, partially, explained by antigenic drift in circulating strains during the season, there is likely a host factor influencing the protection over time. Various reports show antibody titres in vaccine recipients decline over the course of the season, even reaching pre-vaccination levels after one year. To what extent a decline in antibody titre translates to actual insufficient protection remains somewhat speculative, since the relation between antibody titre and protection is not entirely understood and depends on factors such as age, health status and immune competence of the vaccine recipient.
If waning of antibody titres indeed causes reduction in vaccine effectiveness during the influenza season, it is essential that new vaccines induce the most optimal immune responses lasting at protective levels over time.
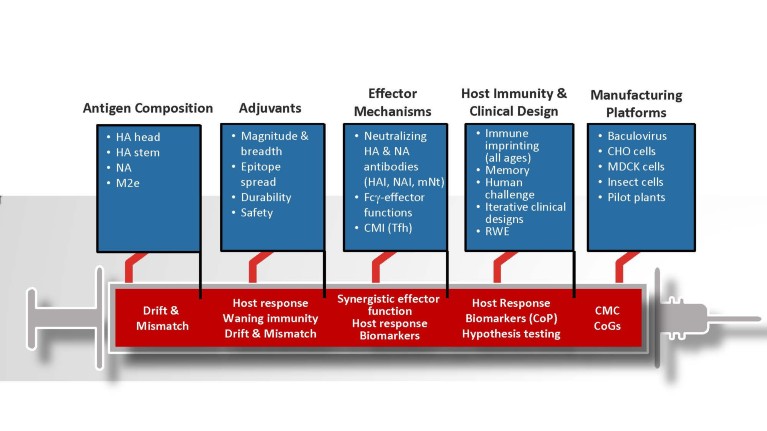
Figure 1. Improving influenza vaccine effectiveness will be driven through research and development across five pillars: antigen composition, adjuvants, effector mechanisms, host immunity and clinical design, and manufacturing platforms. CHO, Chinese hamster ovary cells; CMC, chemistry manufacturing and controls; CMI, cell-mediated immunity; CoGs, cost of goods; CoP, correlates of protection; Fсγ-effector, fragment crystallizable gamma receptor effector; HA, hemagglutinin; HAI, hemagglutinin inhibition; M2e, influenza virus matrix protein 2 ectodomain; MDCK, Madin-Darby canine kidney; mNt, microneutralization test; NA, neuraminidase; NAI, neuraminidase inhibition; RWE, real world evidence; Tfh, T follicular helper cell.
Human host factors
Third, increasing evidence shows that age, immunological fitness, exposure to previous influenza viruses and genetic makeup determine largely how well a certain vaccinee will develop a protective immune response at the outset of vaccination. Why does the influenza attack rate or protection after vaccination vary with age? One hypothesis is the antibody response against a given strain is influenced by the immunological memory induced against the strain or strains encountered for the first time early in life — the so-called phenomenon of ‘original antigenic sin’ or immunological imprinting8. It is also thought this decreased antibody response to subsequent exposures may be a result of ‘antigen trapping’, which means pre-existing, cross-reactive antibodies and memory cells ‘capture’ the new antigen and decrease the antigenic load available for priming naive B cells, which would lead to a diminished novel response9. Seemingly supporting this hypothesis is the observation that longitudinal exposure to antigenically drifting strains is potentially responsible for maintaining a strong memory response against strains encountered early in life10.
Co-morbidity and frailty also impact the host’s ability to mount an adequate vaccine response and contribute to a decrease of immunological fitness11. Immunological fitness is also affected by the intrinsic aging of the immune system itself (known as immunosenescence); by immune immaturity early in life; and by immune naivety to novel antigens early in life and to new pandemic strains. Various factors in the immune response have been reported to diminish in aged individuals. The multiplicity of these impairments, including decreased antibody levels and lowered antibody specificity, is thought to cause a reduction in influenza vaccine efficacy in the elderly.
In younger populations, particularly infants, immaturity of the immune system plays a significant role in suboptimal efficacy of influenza vaccines, which necessitates boosting of the immune response by repeated vaccinations, addition of adjuvants or other means. In newborns and up to 6 months of age it may be particularly difficult to find effective solutions for active immunization. However, maternal immunization has been shown to offer some protection in this age group, thereby closing the gap between birth and receipt of influenza vaccine at 6 months of age.
Thus, many facets, both intrinsic and extrinsic to the recipient, define the three main reasons for suboptimal and variable influenza vaccine effectiveness. An understanding of these mechanisms and finding innovative ways to address them with scientific advances and new vaccine approaches are crucially important to developing improved effectiveness.
Innovating better vaccines for tomorrow
We know influenza remains a wily opponent, with scientists working to address the unpredictability and complexity of each season. To that end, differentiated and improved influenza vaccines, including high-dose vaccines, adjuvanted vaccines and those produced with new technology, such as recombinant protein and cell-based vaccines, are demonstrating greater efficacy within different age groups. To continue its leadership in research and development of improved influenza vaccines, Sanofi Pasteur is focusing its efforts on five key pillars: antigen composition; adjuvants; induction of synergistic immunological effector functions; understanding of host immunity to influenza; and the adoption of next-generation vaccine-manufacturing platforms (Fig. 1). Each pillar has the potential to address the three main reasons behind reduced influenza vaccine effectiveness.
The first pillar addresses vaccine composition, presenting a well-matched hemagglutinin (HA) to the human immune system, and potentially additional antigens with the ability to protect against heterologous viruses, such as the second most abundant glycoprotein, neuraminidase (NA). The importance of HA antigen selection and expression of a structurally intact molecule that targets immunity to conserved epitopes on the globular (HA1) head and the HA2 stalk could ensure greater breadth against evolving seasonal viruses. Eliciting immunity to antigens like NA may confer protection and reduce disease symptoms12. NA has been shown to elicit neutralizing and non-neutralizing antibodies with different immunological effector functions, acting synergistically to enhance protection and reduce disease severity.
The second pillar makes the case for inclusion of an immune enhancer or adjuvant to increase the magnitude of protective immune response and, in turn, address waning immunity. Ensuring antibody titres remain high throughout the influenza season is consistent with sustained protection and increased vaccine effectiveness. Additional attributes are the potential for epitope spread by increasing cross-reactivity to other virus strains, which can have a direct impact on breadth of virus coverage and enhanced cellular immunity13. With few adjuvants being part of today’s licensed influenza vaccines, we aim to conduct more clinical research on novel candidate adjuvants, especially in key target populations such as the young, the elderly and the immunocompromised.
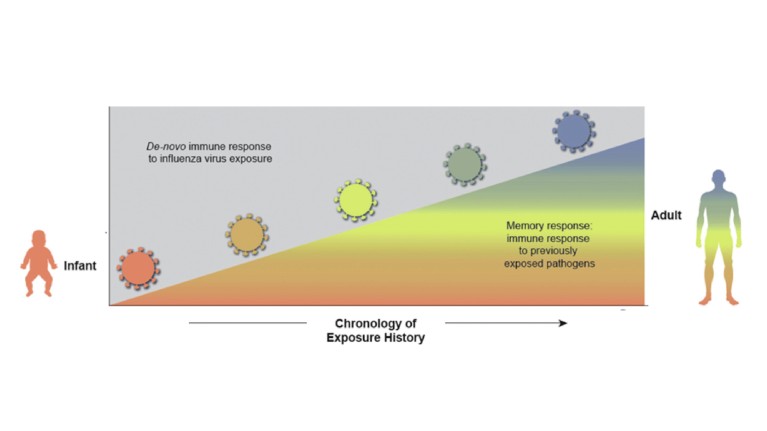
Figure 2. Host immunity influences response to vaccination. Age is an important determinant of the immune response to influenza virus exposure. During life we encounter increasing numbers of different influenza viruses that shape our memory response to new influenza viruses.
The third pillar concerns a shortfall in harnessing alternate effector functions. Today’s licensed vaccines rely predominantly on HA inhibition (HAI) and neutralizing antibodies. While HAI is an established correlate of protection, there is an opportunity to target synergistic effector functions. Classical virology teaches us the importance of neutralizing antibodies in preventing infection, but the roles of Fc-dependent non-neutralizing antibodies targeting both HA and NA are only now being understood and have shown a significant bearing on disease modulation. Therefore, the inclusion of other antigens and the use of an adjuvant that can elicit different immunological effector functions are expected to maximize protection, both at the initial infection stage and during virus spread.
The fourth pillar relies on understanding the influence of the host’s pre-existing influenza immune history on subsequent vaccination. As mentioned, natural exposure to influenza early in life imprints the host and shapes immunity to subsequent vaccination. As a direct consequence, effectiveness has been variable across different age groups, driven by the immunological imprint or exposure of an individual (Fig. 2). Studies have shown, despite original antigenic sin, current vaccines can effectively recall immunity to previously seen strains often resulting in breadth, a phenomenon described as immunological ‘back-boosting’14. Today’s technologies allow us to study immunity in subjects with different immune histories at the cellular level, helping us better understand this phenomenon and ultimately assess performance of improved vaccines. Factoring this information into vaccine design to maximize immunity to key determinants across all age groups is a goal for ‘next-generation’ influenza vaccines.
The fifth pillar looks at the impact of using modern influenza vaccine manufacturing technology, inherently designed to preserve the fidelity of the vaccine sequence and address issues of reduced vaccine effectiveness from adaptation of the virus during passage in eggs or culture cells. Technologies that rely upon production of subunit proteins may have an inherent advantage as they are less prone to change and can be a better match to circulating viruses. Equally, messenger RNA (mRNA)-based technologies hold promise, given its ability to preserve sequence fidelity and deliver a multivalent vaccine formulation that could address the need to cover co-circulating virus drift variants.
Critical to the advancement of influenza vaccine science is the development of tools, models and working platforms to ensure their success. This includes the development of pre-clinical models translatable to the human immune response to influenza and vaccines, optimal selection of vaccine strains, identification of predictive biomarkers beyond the traditional HAI assay, and new immunological assays to evaluate the impact of these approaches on increasing vaccine effectiveness and reducing severe outcomes. When testing novel vaccines in clinical trials, we may have to defer from classical and costly efficacy studies to more adaptive trial designs that will have the benefit of assessing the impact of key variables and their contribution to efficacy, as will a global perspective that embraces the role of real-world evidence.
The journey to tomorrow
The quest for a ‘universal’ influenza vaccine — one vaccination that protects against all human influenza for life — continues to be the highest goal for our work and the global public-health community. However, the technical challenges of developing this type of vaccine are difficult and will likely take decades to overcome. At Sanofi Pasteur, we believe a more achievable, and increasingly impactful, approach is an iterative stepwise development pathway.
As a first step, we aim to build on available influenza vaccines by developing a multi-component vaccine that provides greater effectiveness by limiting the impact of vaccine mismatch through inducing broader ‘strain-specific’ immunity. Such vaccines could reduce low effectiveness seen in some years by providing protection to cover the majority of circulating strains within the four circulating subtypes: influenza A virus subtypes H3N2 and H1N1, and influenza B virus subtypes Yamagata and Victoria. This would build on learnings from current vaccines and seek to engage new mechanisms of protection, antigenic targets, adjuvants and structural biology for antigen design. Such a ‘forward-looking’ vaccine would cover future strains likely to circulate in subsequent seasons.
As a second step, we will look to develop a vaccine targeting conserved regions of each subtype, or lineage, and thus potentially providing long-lasting protection against novel subtypes and lineages with pandemic potential. This approach, which would necessitate multi-components, might provide sufficient protection against the majority of influenza epidemics and potential pandemics.
Our goal is for these two steps to provide iterative information on protective mechanisms to develop a ‘universal’ influenza vaccine that covers both influenza A and influenza B types including those of pandemic potential. This would be particularly important for infants where vaccination would enable ‘imprinting’ of the immune response early in life and could potentially direct the immune response to the highly conserved regions of the virus targeted by the universal influenza vaccine.
In this stepwise approach, a number of challenging questions remain to be answered. Do we expect the same vaccine to work equally well for naive infants, imprinted adults and older immunosenescent populations? How often would the vaccine need to be administered to maintain long-lasting protection — annually versus a booster every five years?
At Sanofi Pasteur, we will continue to ask those questions and aim to enhance the science of influenza immunization through innovative research, development, and delivery of differentiated and proven vaccines. We are committed to raising the public awareness of the serious and far-reaching impact of influenza on human health, and the positive benefits of today’s vaccines, while spearheading efforts to reduce human suffering through our next-generation influenza vaccines.

