The three most common types of brain tumour are intracranial metastases, which spread from other primary sites of disease; meningiomas, which are mostly benign; and glioblastoma multiforme (GBM), which is the most common and lethal primary malignant brain tumour in adults. Median survival in GBM following standard of care treatment is approximately 15 months from the time of diagnosis. There are currently no curative therapeutic options for GBM and treatment includes maximal surgical resection, radiation therapy (RT) and chemotherapy. The effectiveness of these therapies is limited by high rates of tumour recurrence, treatment-related toxicity, emerging resistance to therapy and ongoing neurological deterioration. There are few systemic therapies available for GBM and temozolomide (TMZ) is the preferred first line chemotherapeutic agent, given in combination with RT following surgical resection and later as a maintenance therapy1. Tumor Treating Fields (TTFields) is a new treatment modality for GBM, developed and pioneered by Novocure, a global oncology company. Treatment with TTFields has been proven to provide significant clinical benefit for GBM patients.
The TTFields treatment modality is the brainchild of Professor Yoram Palti (Professor Emeritus of Physiology and Biophysics at the Technion Israel Institute of Technology in Haifa, Israel) who hypothesized that alternating electric fields in the intermediate frequency range could disrupt cancer cell division and cause cancer cell death. Professor Palti rationalized that electric fields within the frequency range of 100–300 kHz would penetrate rapidly dividing cancer cells and disrupt essential processes and cellular structures leading to apoptotic cell death. To test his hypothesis, Professor Palti set up a home laboratory, where he successfully demonstrated that when applied at tumour cell-specific frequencies (200 kHz for GBM), alternating electric fields disrupt cell division, leading to cancer cell death but sparing healthy cells. Encouraged by these results, Novocure was founded in 2000 and has grown into an international oncology company with more than 600 employees and operations in the United States, Europe and Asia. With almost 20 years of continued research, Novocure has met many significant milestones (Fig. 1) and has established itself as an innovator in oncology, dedicated to improving the lives of people with cancer.

Novocure milestones in brain cancer treatment. Novocure was founded in 2000 with a patient-forward approach that remains the centre of its corporate mission. The timeline highlights more than 18 years of preclinical and clinical research with many significant milestones, establishing Novocure as an innovator in oncology and dedicated to improving the lives of people with brain cancer. Optune® is a non-invasive portable device that delivers alternating electric fields to dividing cancer cells. The NovoTAL™ system is a software programme that optimizes transducer array layouts for an individual patient based on head size and tumour location. GBM, glioblastoma multiforme; NCCN, National Comprehensive Cancer Network.
Mechanism of action of TTFields
TTFields are low intensity, intermediate frequency alternating electric fields that act upon rapidly dividing glioma and other cancer cells2,3 especially during metaphase, anaphase and telophase of mitotic cell division. When an alternating electric field is generated across a cancer cell, charged molecules within the cell will move back and forth and dipolar molecules will rotate. At sufficiently high frequencies, the motility of such molecules diminishes. Molecules with a high electrical dipole moment such as tubulin dimers and septins are therefore forced to align with the direction of the alternating electric fields (TTFields) under a uniform field distribution, which is generated in cells during metaphase. This disrupts microtubule spindle formation and septin fibres localization during metaphase, leading to mitotic catastrophe, which may culminate in mitotic cell death. Many of the cells, however, will be able to proceed from metaphase to anaphase and telophase. During these phases, the dividing cell assumes an hourglass shape as it begins to split into two distinct daughter cells, causing a non-uniform alternating electric field. This non-uniform field causes polarized cellular components to migrate towards the cleavage furrow of the two daughter cells (an effect called dielectrophoresis) and the dividing cells are unable to divide properly. Overall, the anti-mitotic effect of TTFields may ultimately lead to cell death or to the formation of abnormal dividing cells with an uneven number of chromosomes (Fig. 2).
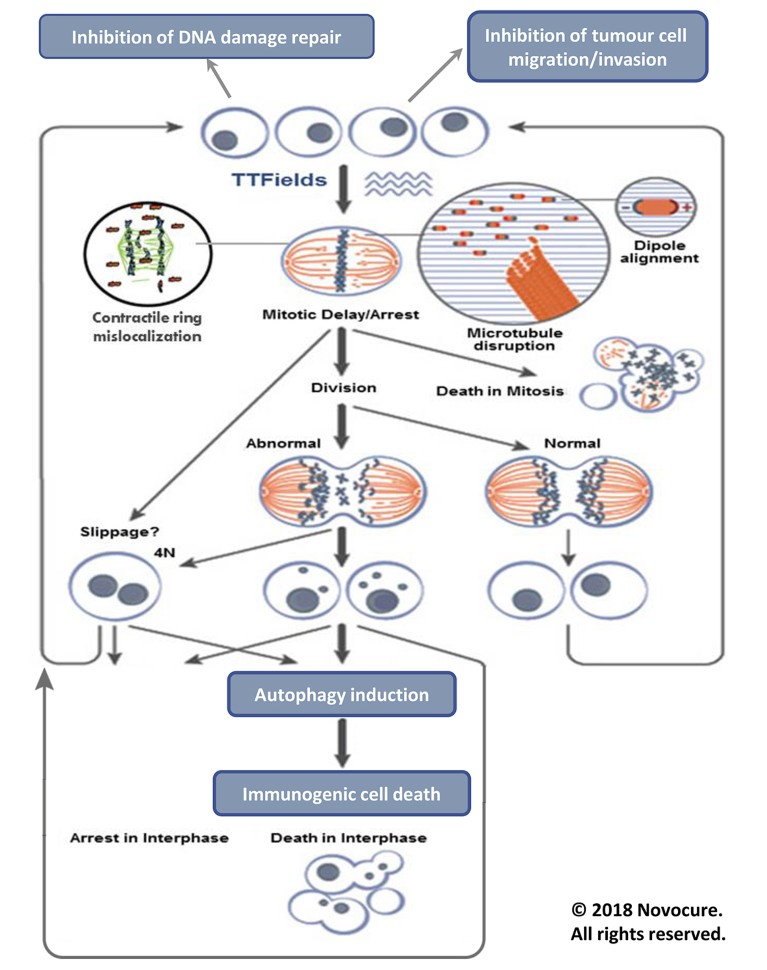
Figure 2: Effects of TTFields on replicating cells. TTFields exert directional forces on polar microtubules and interfere with the assembly of the normal mitotic spindle and subsequently trigger mitotic cell death. TTFields also inhibit DNA damage repair, impair cellular migration and upregulate autophagy, resulting in immunogenic cell death.
Ongoing research suggests that TTFields may also inhibit DNA damage repair, impair cellular migration and invasion4 and upregulate autophagy5. The resulting daughter cells exhibit various forms of cell death including immunogenic cell death, suggesting that combining TTFields with immunotherapies may enhance the body’s own antitumour immunity6. In pre-clinical studies, increased sensitivity to chemotherapy when combined with TTFields was demonstrated in human glioblastoma cell lines and in animal tumour models2,3,7. There is also a reported synergistic effect between TTFields and RT, suggesting that GBM patients may benefit from this combination8.
TTFields treatment - the Optune® delivery system
TTFields are administered to GBM patients using the patient-operated, home-use Optune device, which delivers alternating electric fields through transducer arrays placed on the patient’s shaved scalp. The first and second generation Optune devices are shown in Fig. 3. The second-generation device reflects design improvements intended to improve patients’ experience with TTFields treatment. Weighing approximately 1.2 kg (2.7 lbs), the lightweight design allows patients to perform normal daily activities while receiving treatment (Fig. 3).

Figure 3: First and second generation Optune devices. The first and second generation Optune devices are comprised of two primary components: the electric field generator, preset to 200 kHz for GBM, and insulated transducer arrays incorporated in four bandages. The device treatment kit includes a plug-in power supply, portable battery, battery rack, battery charger, connecting cables and carrying case. Design changes in the second generation device using improved electronic components, circuit boards and digital signaling technology, resulted in reduced weight and increased operational efficiency for people with glioblastoma multiforme, improving the patient experience with TTFields treatment. (Top left, 1st generation Optune; Top Right, 2nd generation Optune; Bottom panels, patients wearing 2nd generation Optune with white and tan arrays).
The Optune system consists of two primary components – the electric field generator and two pairs of transducer arrays, which deliver the fields non-invasively to the tumour site. Further design enhancements include the availability of tan coloured arrays which are less conspicuous. For cosmetic reasons, patients may conceal the arrays beneath a scarf, hat or a wig. The portable field generator can operate from the mains electricity supply or from a rechargeable battery.
Precise placement of the transducer arrays is important to optimize the clinical effect of TTFields. Novocure has developed the NovoTAL software system to optimize array layouts based on individual patient head size, tumour location and magnetic resonance imaging data for the specific characteristics of the patient’s tumour9. Preclinical studies show that the effects of TTFields increase with intensity, which underscores the critical need to understand how TTFields intensities distribute within the tumour region. There are no practical means for measuring field intensities within the brain tissue and tumours of patients undergoing treatment. Simulations and modelling are the primary tools for obtaining these essential data (Fig. 4). Simulation-based studies using realistic head models have shown that TTFields effectively penetrate the brain and tumour tissue. The field distribution is heterogeneous and depends on the anatomy of the individual patient, the physical properties of the various tissue types and the location of the tumour10. The position of arrays can therefore be optimized using the NovoTAL System to deliver maximal field intensities to the tumour region of the individual patient10,11.
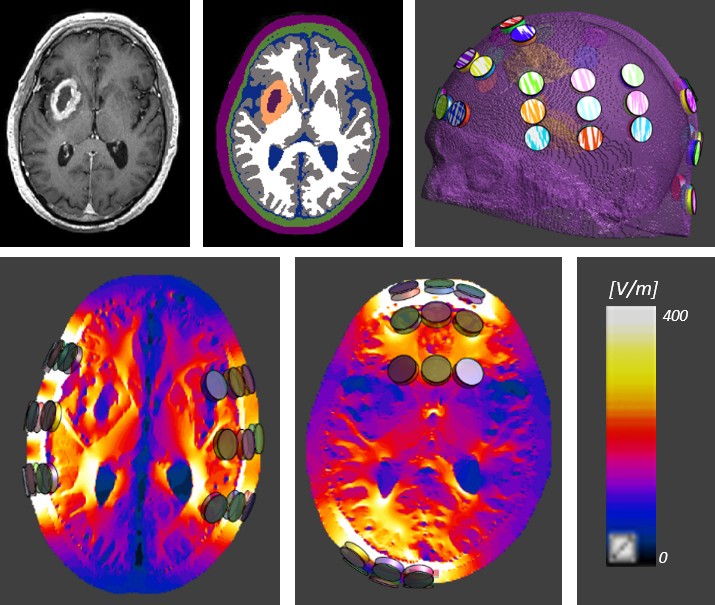
Figure 4: Head phantom with transducers and simulated Tumor Treating Fields intensity in brain tissue. Simulation-based studies using a realistic head model show that TTFieldseff ectively penetrate brain tissues and that the electric fi eld intensity distribution is heterogeneous. Panels in the top row show axial slices of a T1-contrast MRI of a GBM patient(top-left), and a realistic model used to numerically simulate delivery of TTFields to the patient (top-middle and right). Bottom panels show the field distributions created by the leftright (bottom- left) and anterior-posterior (bottom-middle) pairs of arrays. The array layout is optimized to deliver higher field intensities to the region of the tumour.
Clinical development of TTFields for Glioblastoma multiforme
Early pre-clinical data demonstrated that TTFields arrest cell proliferation in animal tumour models and had an additive treatment effect when combined with chemotherapy2,3 The encouraging results of a first-in-man study in various cancers led to the initiation of a pilot clinical trial (EF-07) of 20 recurrent and newly diagnosed GBM patients in 2004 that validated the feasibility of treating GBM with TTFields2,3. Four of the patients from the pilot study are still alive12. The subsequent phase III EF-11 clinical trial demonstrated the efficacy and safety of TTFields for recurrent GBM13 resulting in United States Food and Drug Administration (FDA) approval in 2011.
Stupp et al. in 20051 reported what is still to this day considered the definitive protocol for the treatment of newly diagnosed GBM – sometimes referred to as the Stupp protocol. Following maximal safe surgical tumour removal, the patient received RT plus TMZ followed by TMZ maintenance therapy. Compared to RT therapy alone, the combination of RT and TMZ significantly increased the median overall survival for GBM patients by 2.5 months (median overall survival 12.1 months and 14.6 months, respectively) and the two-year survival rate was 10.4% for RT alone compared to 26.5% for GBM patients in the RT plus TMZ treatment group. At the time these results were groundbreaking.
Novocure launched a second phase III clinical trial (EF-14) for newly diagnosed GBM to test the efficacy and safety of TTFields in combination with maintenance TMZ. In 2015 and 2017, Stupp et al. published the interim14 and final results15 of the phase III EF-14 study, respectively, that showed that adding TTFields to the maintenance phase of the Stupp protocol further improved progression free survival (PFS) and overall survival (OS) in newly diagnosed GBM patients. The addition of TTFields to TMZ maintenance therapy significantly increased OS by 4.9 months compared to patients receiving TMZ alone (20.9 months and 16.0 months, respectively)15. Patients in the EF-14 study had already undergone maximal surgical resection followed by RT plus TMZ prior to enrollment, and the median time from diagnosis to randomization was 3.8 months for both groups. Therefore, the median OS for the TTFields plus TMZ group was 24.7 months from the time of diagnosis. The two-year and five-year survival rates from randomization for patients receiving TTFields plus TMZ were 43% and 13%, respectively, compared to 31% and 5% for patients receiving TMZ alone. These data led to the FDA approval of TTFields therapy combined with TMZ for the treatment of newly diagnosed GBM patients in 2015. The significance of these results is reflected by the National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines Category 1 recommendation for TTFields plus TMZ as a standard treatment option for newly diagnosed GBM16. A recent analysis that applied an integrated survival model to the EF-14 study results17 indicated that patients treated with TTFields plus TMZ had an incremental lifetime survival of 1.8 years (TTFields plus TMZ, 4.2 years vs TMZ alone, 2.4 years). Patients alive at year 2 after commencing treatment with TTFields had a 20.7% probability of surviving to year 10.
TTFields’ unique mechanism of action underscores key points related to its effective administration and clinical benefit. Unlike chemotherapeutic agents, TTFields are only active while the arrays are affixed to the scalp and the alternating electric fields are generated between the transducer arrays. As a loco-regional and non-invasive targeted therapy, TTFields has the benefit of avoiding systemic adverse events associated with chemotherapy and targeted systemic therapies. The primary treatment-associated adverse event experienced by some patients with TTFields is skin irritation below the arrays, which is predictable and easily manageable in the majority of cases. The lack of systemic adverse events allows TTFields to be potentially combined with other therapeutic modalities with some assurance that TTFields treatment may offer synergistic clinical benefit with targeted treatments without compounding adverse systemic effects. Health-related quality of life (HRQoL) is a prominent concern in treating brain tumours, and the combination of TTFields with TMZ had no negative influence on HRQoL for GBM patients with the exception of itchy skin18, an expected consequence of the long-term application of the transducer arrays to the patient’s shaved scalp. In fact, the longer PFS observed in TTFields-treated patients was accompanied by a longer time to progression related deterioration in several important HRQoL scales.
Unlike systemic cancer therapies, TTFields only act against rapidly dividing cancer cells while the transducer arrays are adhered to the scalp and TTFields are active. Consequently, the average daily usage of the device (or treatment compliance) is a crucial component of clinical benefit. The phase III GBM studies demonstrated a survival advantage for patients with a maximal monthly compliance rate of ≥75%. Further analyses showed that survival outcomes are enhanced starting at >50% compliance and that patients achieving 90% compliance show maximal benefit at five years with 29.3% of patients still alive19.
Ongoing TTFields research in brain cancer and other tumour types
TTFields is an innovative treatment modality approved for both newly diagnosed and recurrent GBM in US, Europe and Japan. The mechanism of action has relevance in other cancers. Novocure continues to explore the use of TTFields in a number of cancers of the central nervous system including brain metastases from non-small cell lung cancer (NSCLC) in the ongoing phase III METIS trial. Based on the treatment success in GBM, TTFields are being investigated in a number of other solid tumours outside of the brain20. Phase II clinical trials have been completed in mesothelioma, ovarian cancer, NSCLC and pancreatic adenocarcinoma. Phase III trials are underway in pancreatic adenocarcinoma and NSCLC. The FDA has designated the TTFields delivery system as a humanitarian use device for the treatment of pleural mesothelioma.
Patients remain at the heart of the work at Novocure, guiding us forward in our goal to deliver a novel, safe and efficacious cancer therapy that prolongs survival while maintaining patient quality of life.


 Nature Outlook: Brain cancer
Nature Outlook: Brain cancer