Abstract
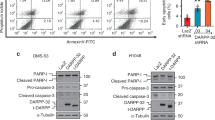
Carcinoids are neuroendocrine neoplasms that cause significant morbidity and mortality and for which few effective therapies are available. Given the recent identification of the anticancer flavonoid chrysin, we sought to investigate its therapeutic potential in carcinoids. Here we report chrysin’s ability to modulate the achaete-scute complex-like 1 (ASCL1), a neuroendocrine-specific transcription factor highly implicated in the malignant phenotype of carcinoids and other neuroendocrine cancers. Moreover, we elucidate the role of ASCL1 in carcinoid growth and bioactivity. Treatment of two carcinoid cell lines (BON and H727) with varying chrysin concentrations suppressed cell proliferation, while reducing expression of ASCL1 and the neuroendocrine biomarker chromogranin A (CgA), demonstrated by western blotting. Propidium iodide and phycoerythrin AnnexinV/7-aminoactinomycin D staining and sorting following chrysin treatment revealed S/G2 phase arrest and apoptosis, respectively. This was corroborated by chrysin-induced cleavage of caspase-3 and poly ADP-ribose polymerase and activation of p21Waf1/Cip1. Furthermore, direct ASCL1 knockdown with an ASCL1-specific small interfering RNA inhibited CgA and synaptophysin expression as well as carcinoid proliferation, while also reducing cyclin B1 and D1 and increasing p21Waf1/Cip1 and p27Kip1 expression, suggesting an arrest of the cell cycle. Collectively, these findings warrant the deliberation of targeted ASCL1 suppression by chrysin or other agents as a therapeutic approach for carcinoid management.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Oberg K . Genetics and molecular pathology of neuroendocrine gastrointestinal and pancreatic tumors (gastroenteropancreatic neuroendocrine tumors). Curr Opin Endocrinol Diabetes Obes 2009; 16: 72–78.
Sippel RS, Chen H . Carcinoid tumors. Surg Oncol Clin N Am 2006; 15: 463–478.
Calender A . Genetics of neuroendocrine tumors. Rev Prat 2002; 52: 256–261.
Maggard MA, O'Connell JB, Ko CY . Updated population-based review of carcinoid tumors. Ann Surg 2004; 240: 117–122.
Thomas CF, Tazelaar HD, Jett JR . Typical and atypical pulmonary carcinoids: outcome in patients presenting with regional lymph node involvement. Chest 2001; 119: 1143–1150.
Maroun J, Kocha W, Kvols L, Bjarnason G, Chen E, Germond C et al. Guidelines for the diagnosis and management of carcinoid tumours. Part 1: the gastrointestinal tract. A statement from a Canadian National Carcinoid Expert Group. Curr Oncol 2006; 13: 67–76.
Pasieka JL . Carcinoid tumors. Surg Clin North Am 2009; 89: 1123–1137.
Chen H, Hardacre J, Uzar A, Cameron J, Choti M . Isolated liver metastases from neuroendocrine tumors: does resection prolong survival? J Am Coll Surg 1998; 187: 88–92 discussion 92-3.
Zuetenhorst JM, Taal BG . Metastatic carcinoid tumors: a clinical review. Oncologist 2005; 10: 123–131.
Modlin IM, Latich I, Kidd M, Zikusoka M, Eick G . Therapeutic options for gastrointestinal carcinoids. Clin Gastroenterol Hepatol 2006; 4: 526–547.
de Herder WW, Hofland LJ, van der Lely AJ, Lamberts SW . Somatostatin receptors in gastroentero-pancreatic neuroendocrine tumours. Endocr Relat Cancer 2003; 10: 451–458.
Borges M, Linnoila RI, van de Velde HJ, Chen H, Nelkin BD, Mabry M et al. An achaete-scute homologue essential for neuroendocrine differentiation in the lung. Nature 1997; 386: 852–855.
Nakakura E, Sriuranpong V, Kunnimalaiyaan M, Hsiao E, Schuebel K, Borges M et al. Regulation of neuroendocrine differentiation in gastrointestinal carcinoid tumor cells by notch signaling. J Clin Endocrinol Metab 2005; 90: 4350–4356.
Sippel R, Carpenter J, Kunnimalaiyaan M, Chen H . The role of human achaete-scute homolog-1 in medullary thyroid cancer cells. Surgery 2003; 134: 866–871; discussion 871–873.
Jiang S, Kameya T, Asamura H, Umezawa A, Sato Y, Shinada J et al. hASH1 expression is closely correlated with endocrine phenotype and differentiation extent in pulmonary neuroendocrine tumors. Mod Pathol 2004; 17: 222–229.
Chen H, Biel MA, Borges MW, Thiagalingam A, Nelkin BD, Baylin SB et al. Tissue-specific expression of human achaete-scute homologue-1 in neuroendocrine tumors: transcriptional regulation by dual inhibitory regions. Cell Growth Differ 1997; 8: 677–686.
Shida T, Furuya M, Kishimoto T, Nikaido T, Tanizawa T, Koda K et al. The expression of NeuroD and mASH1 in the gastroenteropancreatic neuroendocrine tumors. Mod Pathol 2008; 21: 1363–1370.
Ball DW, Azzoli CG, Baylin SB, Chi D, Dou S, Donis-Keller H et al. Identification of a human achaete-scute homolog highly expressed in neuroendocrine tumors. Proc Natl Acad Sci USA 1993; 90: 5648–5652.
Chen H, Udelsman R, Zeiger M, Ball D . Human achaete-scute homolog-1 is highly expressed in a subset of neuroendocrine tumors. Oncol Rep 1997; 4: 775–778.
Carney ME, O'Reilly RC, Sholevar B, Buiakova OI, Lowry LD, Keane WM et al. Expression of the human Achaete-scute 1 gene in olfactory neuroblastoma (esthesioneuroblastoma). J Neurooncol 1995; 26: 35–43.
Guillemot F, Joyner AL . Dynamic expression of the murine Achaete-Scute homologue Mash-1 in the developing nervous system. Mech Dev 1993; 42: 171–185.
Guillemot F, Nagy A, Auerbach A, Rossant J, Joyner AL . Essential role of Mash-2 in extraembryonic development. Nature 1994; 371: 333–336.
Lo L, Guillemot F, Joyner AL, Anderson DJ . MASH-1: a marker and a mutation for mammalian neural crest development. Perspect Dev Neurobiol 1994; 2: 191–201.
Lanigan TM, DeRaad SK, Russo AF . Requirement of the MASH-1 transcription factor for neuroendocrine differentiation of thyroid C cells. J Neurobiol 1998; 34: 126–134.
Sippel RS, Carpenter JE, Kunnimalaiyaan M, Lagerholm S, Chen H . Raf-1 activation suppresses neuroendocrine marker and hormone levels in human gastrointestinal carcinoid cells. Am J Physiol Gastrointest Liver Physiol 2003; 285: G245–G254.
Kunnimalaiyaan M, Traeger K, Chen H . Conservation of the Notch1 signaling pathway in gastrointestinal carcinoid cells. Am J Physiol Gastrointest Liver Physiol 2005; 289: G636–G642.
Pitt SC, Chen H, Kunnimalaiyaan M . Inhibition of phosphatidylinositol 3-kinase/Akt signaling suppresses tumor cell proliferation and neuroendocrine marker expression in GI carcinoid tumors. Ann Surg Oncol 2009; 16: 2936–2942.
Nakakura EK, Sriuranpong VR, Kunnimalaiyaan M, Hsiao EC, Schuebel KE, Borges MW et al. Regulation of neuroendocrine differentiation in gastrointestinal carcinoid tumor cells by notch signaling. J Clin Endocrinol Metab 2005; 90: 4350–4356.
Kunnimalaiyaan M, Vaccaro AM, Ndiaye MA, Chen H . Overexpression of the NOTCH1 intracellular domain inhibits cell proliferation and alters the neuroendocrine phenotype of medullary thyroid cancer cells. J Biol Chem 2006; 281: 39819–39830.
Yoon K, Gaiano N . Notch signaling in the mammalian central nervous system: insights from mouse mutants. Nat Neurosci 2005; 8: 709–715.
Maillard I, Pear WS . Notch and cancer: best to avoid the ups and downs. Cancer Cell 2003; 3: 203–205.
Sriuranpong V, Borges MW, Strock CL, Nakakura EK, Watkins DN, Blaumueller CM et al. Notch signaling induces rapid degradation of achaete-scute homolog 1. Mol Cell Biol 2002; 22: 3129–3139.
Sriuranpong V, Borges MW, Ravi RK, Arnold DR, Nelkin BD, Baylin SB et al. Notch signaling induces cell cycle arrest in small cell lung cancer cells. Cancer Res 2001; 61: 3200–3205.
de la Pompa JL, Wakeham A, Correia KM, Samper E, Brown S, Aguilera RJ et al. Conservation of the Notch signalling pathway in mammalian neurogenesis. Development 1997; 124: 1139–1148.
Jensen J, Pedersen EE, Galante P, Hald J, Heller RS, Ishibashi M et al. Control of endodermal endocrine development by Hes-1. Nat Genet 2000; 24: 36–44.
Apelqvist A, Li H, Sommer L, Beatus P, Anderson DJ, Honjo T et al. Notch signalling controls pancreatic cell differentiation. Nature 1999; 400: 877–881.
Monasterio A, Urdaci MC, Pinchuk IV, López-Moratalla N, Martínez-Irujo JJ . Flavonoids induce apoptosis in human leukemia U937 cells through caspase- and caspase-calpain-dependent pathways. Nutr Cancer 2004; 50: 90–100.
Woo KJ, Jeong YJ, Park JW, Kwon TK . Chrysin-induced apoptosis is mediated through caspase activation and Akt inactivation in U937 leukemia cells. Biochem Biophys Res Commun 2004; 325: 1215–1222.
Yu XM, Phan T, Patel PN, Jaskula-Sztul R, Chen H . Chrysin activates Notch1 signaling and suppresses tumor growth of anaplastic thyroid carcinoma in vitro and in vivo. Cancer 2013; 119: 774–781.
Khoo BY, Chua SL, Balaram P . Apoptotic effects of chrysin in human cancer cell lines. Int J Mol Sci 2010; 11: 2188–2199.
Pal-Bhadra M, Ramaiah MJ, Reddy TL, Krishnan A, Pushpavalli SN, Babu KS et al. Plant HDAC inhibitor chrysin arrest cell growth and induce p21WAF1 by altering chromatin of STAT response element in A375 cells. BMC Cancer 2012; 12: 180.
Weng MS, Ho YS, Lin JK . Chrysin induces G1 phase cell cycle arrest in C6 glioma cells through inducing p21Waf1/Cip1 expression: involvement of p38 mitogen-activated protein kinase. Biochem Pharmacol 2005; 69: 1815–1827.
Zhang Q, Zhao XH, Wang ZJ . Cytotoxicity of flavones and flavonols to a human esophageal squamous cell carcinoma cell line (KYSE-510) by induction of G2/M arrest and apoptosis. Toxicol In Vitro 2009; 23: 797–807.
Wang W, VanAlstyne PC, Irons KA, Chen S, Stewart JW, Birt DF . Individual and interactive effects of apigenin analogs on G2/M cell-cycle arrest in human colon carcinoma cell lines. Nutr Cancer 2004; 48: 106–114.
Woodman OL, Chan ECh . Vascular and anti-oxidant actions of flavonols and flavones. Clin Exp Pharmacol Physiol 2004; 31: 786–790.
Middleton E, Kandaswami C, Theoharides TC . The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol Rev 2000; 52: 673–751.
Cook MR, Pinchot SN, Jaskula-Sztul R, Luo J, Kunnimalaiyaan M, Chen H . Identification of a novel Raf-1 pathway activator that inhibits gastrointestinal carcinoid cell growth. Mol Cancer Ther 2010; 9: 429–437.
Wyche TP, Dammalapati A, Cho H, Harrison AD, Kwon GS, Chen H et al. Thiocoraline activates the Notch pathway in carcinoids and reduces tumor progression in vivo. Cancer Gene Ther 2014; 21: 518–525.
Greenblatt DY, Cayo M, Ning L, Jaskula-Sztul R, Haymart M, Kunnimalaiyaan M et al. Suberoyl bishydroxamic acid inhibits cellular proliferation by inducing cell cycle arrest in carcinoid cancer cells. J Gastrointest Surg 2007; 11: 1515–1520; discussion 1520.
Pinchot SN, Jaskula-Sztul R, Ning L, Peters NR, Cook MR, Kunnimalaiyaan M et al. Identification and validation of Notch pathway activating compounds through a novel high-throughput screening method. Cancer 2011; 117: 1386–1398.
Lukas J, Bartkova J, Bartek J . Convergence of mitogenic signalling cascades from diverse classes of receptors at the cyclin D-cyclin-dependent kinase-pRb-controlled G1 checkpoint. Mol Cell Biol 1996; 16: 6917–6925.
Sherr CJ, Roberts JM . CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev 1999; 13: 1501–1512.
Cheng M, Olivier P, Diehl JA, Fero M, Roussel MF, Roberts JM et al. The p21(Cip1) and p27(Kip1) CDK 'inhibitors' are essential activators of cyclin D-dependent kinases in murine fibroblasts. EMBO J 1999; 18: 1571–1583.
Gautier J, Solomon MJ, Booher RN, Bazan JF, Kirschner MW . cdc25 is a specific tyrosine phosphatase that directly activates p34cdc2. Cell 1991; 67: 197–211.
Atherton-Fessler S, Liu F, Gabrielli B, Lee MS, Peng CY, Piwnica-Worms H . Cell cycle regulation of the p34cdc2 inhibitory kinases. Mol Biol Cell 1994; 5: 989–1001.
Norbury C, Blow J, Nurse P . Regulatory phosphorylation of the p34cdc2 protein kinase in vertebrates. EMBO J 1991; 10: 3321–3329.
Chen JY, Cook MR, Pinchot SN, Kunnimalaiyaan M, Chen H . MG-132 inhibits carcinoid growth and alters the neuroendocrine phenotype. J Surg Res 2010; 158: 15–19.
Pitt S, Chen H, Kunnimalaiyaan M . Phosphatidylinositol 3-kinase-Akt signaling in pulmonary carcinoid cells. J Am Coll Surg 2009; 209: 82–88.
Somnay Y, Simon K, Harrison AD, Kunnimalaiyaan S, Chen H, Kunnimalaiyaan M . Neuroendocrine phenotype alteration and growth suppression through apoptosis by MK-2206, an allosteric inhibitor of AKT, in carcinoid cell lines in vitro. Anticancer Drugs 2013; 24: 66–72.
Seregni E, Ferrari L, Bajetta E, Martinetti A, Bombardieri E . Clinical significance of blood chromogranin A measurement in neuroendocrine tumours. Ann Oncol 2001; 12 (Suppl 2): S69–S72.
Tomassetti P, Migliori M, Simoni P, Casadei R, De Iasio R, Corinaldesi R et al. Diagnostic value of plasma chromogranin A in neuroendocrine tumours. Eur J Gastroenterol Hepatol 2001; 13: 55–58.
Chejfec G, Falkmer S, Grimelius L, Jacobsson B, Rodensjo M, Wiedenmann B et al. Synaptophysin. A new marker for pancreatic neuroendocrine tumors. Am J Surg Pathol 1987; 11: 241–247.
Lloyd RV, Erickson LA, Jin L, Kulig E, Qian X, Cheville JC et al. p27kip1: a multifunctional cyclin-dependent kinase inhibitor with prognostic significance in human cancers. Am J Pathol 1999; 154: 313–323.
Lorca T, Labbé JC, Devault A, Fesquet D, Capony JP, Cavadore JC et al. Dephosphorylation of cdc2 on threonine 161 is required for cdc2 kinase inactivation and normal anaphase. EMBO J 1992; 11: 2381–2390.
Campuzano S, Carramolino L, Cabrera CV, Ruíz-Gómez M, Villares R, Boronat A et al. Molecular genetics of the achaete-scute gene complex of D. melanogaster. Cell 1985; 40: 327–338.
Sommer L, Shah N, Rao M, Anderson DJ . The cellular function of MASH1 in autonomic neurogenesis. Neuron 1995; 15: 1245–1258.
Greenblatt DY, Vaccaro AM, Jaskula-Sztul R, Ning L, Haymart M, Kunnimalaiyaan M et al. Valproic acid activates notch-1 signaling and regulates the neuroendocrine phenotype in carcinoid cancer cells. Oncologist 2007; 12: 942–951.
Zhang Q, Zhao XH, Wang ZJ . Flavones and flavonols exert cytotoxic effects on a human oesophageal adenocarcinoma cell line (OE33) by causing G2/M arrest and inducing apoptosis. Food Chem Toxicol 2008; 46: 2042–2053.
Zhou P, Jiang W, Zhang Y, Kahn S, Schieren I, Santella R et al. Antisense to cyclin D1 inhibits growth and reverses the transformed phenotype of human esophageal cancer cells. Oncogene 1995; 11: 571–580.
Arber N, Doki Y, Han E, Sgambato A, Zhou P, Kim N et al. Antisense to cyclin D1 inhibits the growth and tumorigenicity of human colon cancer cells. Cancer Res 1997; 57: 1569–1574.
Russell A, Thompson MA, Hendley J, Trute L, Armes J, Germain D . Cyclin D1 and D3 associate with the SCF complex and are coordinately elevated in breast cancer. Oncogene 1999; 18: 1983–1991.
Lukas J, Müller H, Bartkova J, Spitkovsky D, Kjerulff AA, Jansen-Dürr P et al. DNA tumor virus oncoproteins and retinoblastoma gene mutations share the ability to relieve the cell's requirement for cyclin D1 function in G1. J Cell Biol 1994; 125: 625–638.
Liu H, Liu K, Huang Z, Park CM, Thimmegowda NR, Jang JH et al. A chrysin derivative suppresses skin cancer growth by inhibiting cyclin-dependent kinases. J Biol Chem 2013; 288: 25924–25937.
Acknowledgements
This research was supported under the NIH National Research Service Award T32 GM07215 (to YRS), the Howard Hughes Medical Institute Medical Research Fellows Program (to YRS), a research scholarship from the American College of Surgeons (to BZD), a NIH grant R01 CA121115 (to HC), the American Cancer Society MEN2 Thyroid Cancer Professorship 120319-RPM-11-080-01-TBG and Research Scholar Award RSGM TBE-121413 (to HC) and the Layton F. Rikkers, MD, Chair in Surgical Leadership Professorship (to HC).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Somnay, Y., Dull, B., Eide, J. et al. Chrysin suppresses achaete-scute complex-like 1 and alters the neuroendocrine phenotype of carcinoids. Cancer Gene Ther 22, 496–505 (2015). https://doi.org/10.1038/cgt.2015.49
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cgt.2015.49
This article is cited by
-
Emerging cellular and molecular mechanisms underlying anticancer indications of chrysin
Cancer Cell International (2021)
-
Chrysin and its emerging antineoplastic effects
Cancer Gene Therapy (2016)