Abstract
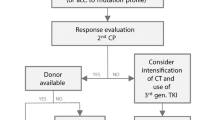
Currently, 50% of adolescents with ALL are treated by adult teams and 50% by paediatric teams (following either adult or paediatric protocols). The aim of this paper is to review the results obtained with first-line chemotherapy and with haematopoietic SCT (HSCT) in adolescents with ALL. Disease biology and host factors are responsible for the differences observed between adolescents and other age categories. The outcome of adolescents with ALL after first-line chemotherapy is poorer as compared with children, although better as compared with adults. Recent studies have shown that adolescents who were enrolled in paediatric trials achieved better results than those who were enrolled in adult trials. This is most likely because of several differences, including protocol design, dose intensity and use of HSCTs, as well as better compliance to treatment and better supportive care. Disparities in the attitude towards treatment between paediatric and adult wards might also contribute to the better outcome that is observed in paediatric institutions. Indications for HSCT in children with ALL are well defined by international protocols. Only very high-risk paediatric patients are eligible for HSCT in CR1, whereas in adult trials, allogeneic or autologous HSCT are frequently offered, even to standard-risk patients in CR1. The outcome of adolescents given HSCT is poorer than in children, though better than in adults. Improving both psychosocial support during therapy and physical exercise habits represent further challenges for teams involved in the treatment of adolescents. Cooperation between paediatric and adult haematologists would surely improve the ability to recruit as many patients as possible and would promote progress in the research on adolescents. In conclusion, redefining age limits according to risk-based strategies, as well as encouraging multi-centre cooperation, should be taken into consideration to improve the outcome of this age category. Adolescents should be referred to research treatment teams that have experience in the management of paediatric ALL and they should be enrolled in international cooperative studies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Pui CH, Schrappe M, Ribeiro RC, Niemeyer CM . Childhood and adolescent lymphoid and myeloid leukemia. Hematology Am Soc Hematol Educ Program 2004; 118–145.
Chessells JM, Hall E, Prentice HG, Durrant J, Bailey CC, Richards SM . The impact of age on outcome in lymphoblastic leukaemia; MRC UKALL X and XA compared: a report from the MRC Paediatric and Adult Working Parties. Leukemia 1998; 12: 463–473.
Boissel N, Auclerc MF, Lhèritier V, Perel Y, Thomas X, Leblanc T et al. Should adolescents with acute lymphoblastic leukemia be treated as old children or young adults? Comparison of the French FRALLE-93 and LALA-94 trials. J Clin Oncol 2003; 21: 774–780.
Reynolds BC, Windebank KP, Leonard RC, Wallace WH . A comparison of self-reported satisfaction between adolescents treated in a ‘teenage’ unit with those treated in adult or paediatric units. Pediatr Blood Cancer 2005; 44: 259–263.
Liu L, Krailo M, Reaman GH, Bernstein L, Surveillance, Epidemiology and End Results Childhood Cancer Linkage Group. Childhood cancer patient's access to cooperative group cancer programs: a population-based study. Cancer 2003; 97: 1339–1345.
Albritton KH, Wiggins CH, Nelson HE, Weeks JC . Site of oncologic specialty care for older adolescents in Utah. J Clin Oncol 2007; 25: 4616–4621.
Conter V, Aricò M, Valsecchi MG, Basso G, Biondi A, Madon E et al. Long-term results of the Italian Association of Pediatric Hematology and Oncology (AIEOP) acute lymphoblastic leukemia studies, 1982–1995. Leukemia 2000; 14: 2196–2204.
De Angelo DJ . The treatment of adolescents and young adults with acute lymphoblastic leukaemia. Hematology Am Soc Hematol Educ Program 2005; 123–130.
Maung ZT, Reid MM, Matheson E, Taylor PR, Proctor SJ, Hall AG . Corticosteroid resistance is increased in lymphoblasts from adults compared to children: preliminary results of in vitro drug sensitivity study in adults with acute lymphoblastic leukaemia. Br J Haematol 1995; 91: 93–100.
Styczynski J, Pieters R, Huismans DR, Schuurhuis GJ, Wysocki M, Veerman AJ . In vitro drug resistance profiles of adult versus childhood acute lymphoblastic leukemia. Br J Haematol 2000; 110: 813–818.
Jeha S . Who should be treating adolescents and young adults with acute lymphoblastic leukaemia? Eur J Cancer 2003; 39: 2579–2583.
Hussein KK, Dahlberg S, Head D, Waddell L, Dabich L, Weick JH et al. Treatment of acute lymphoblastic leukemia in adults with intensive induction, consolidation, and maintenance chemotherapy. Blood 1989; 73: 57–63.
Ramanujachar R, Richards S, Hann I, Goldstone A, Mitchell C, Vora A et al. Adolescents with acute lymphoblastic leukaemia: outcome on UK National Paediatric (ALL 97) and Adult (UKALL XII/E2993) trials. Pediatr Blood Cancer 2007; 48: 254–261.
Ramanujachar R, Richards S, Hann I, Webb D . Adolescents with acute lymphoblastic leukaemia: emerging from the shadow of paediatric and adult treatment protocols. Pediatr Blood Cancer 2006; 47: 748–756.
Testi AM, Valsecchi MG, Conter V, Vignetti M, Nigro LL, Locatelli F et al. Differences in outcome of adolescents with acute lymphoblastic leukaemia enrolled in paediatric (AIEOP) and adult (GIMEMA) protocols. Blood 2004; 104: 539a.
De Bont JM, Holt B, Dekker AW, vanderDoes-vanderBerg A, Sonneveld P, Pieters R et al. Significant difference in outcome for adolescents with acute lymphoblastic leukaemia treated on paediatric vs adult protocols in the Nederlands. Leukemia 2004; 18: 2032–2035.
Nachman J, Sather HN, Buckley JD, Gaynon PS, Steinherz PG, Tubergen DG et al. Young adults 16–21 years of age at diagnosis entered on Children's Cancer Group acute lymphoblastic leukaemia and acute myeloblastic leukaemia protocols. Results of treatment. Cancer 1993; 71: 3377–3385.
Riehm H, Gedner H, Henze G, Kornhuber B, Lampert F, Niethammer D et al. Results and significance of six randomized trials in four consecutive ALL-BFM studies. Hamatol Bluttransfus 1990; 33: 439–450.
Tubergen DG, Gilchrist GS, O’Brien RT, Coccia PF, Sather HN, Waskerwitz MJ et al. Improved outcome with delayed intensification for children with acute lymphoblastic leukaemia and intermediate features: A Children's Cancer Group phase III trial. J Clin Oncol 1993; 11: 527–537.
Silverman LB, Gelber RD, Dal ton VK, Asselin BL, Barr RD, Clavell LA et al. Improved outcome for children with acute lymphoblastic leukemia: results of Dana-Farber Consortium Protocol 91–01. Blood 2000; 97: 1211–1218.
Rubnitz JE, Lensing S, Zhou Y, Sandlund JT, Razzouk BI, Ribeiro RC et al. Death during induction therapy and first remission of acute leukemia in childhood: the St Jude experience. Cancer 2004; 101: 1677–1684.
Schiffer CA . Differences in outcome in adolescents with acute lymphoblastic leukaemia: a consequence of better regimens? Better doctors? Both? J Clin Oncol 2003; 21: 760–761.
Bleyer A . Older adolescents with cancer in North America deficits in outcome and research. Pediatr Clin North Am 2002; 49: 1027–1042.
Peters C, Schrander A, Schrappe M, von Stackelberg A, Stary J, Yaniv I et al. Allogenic haemopoietic stem cell transplantation in children with acute lymphoblastic leukaemia: the BFM/IBFM/EBMT concepts. Bone Marrow Transplant 2005; 35: S9–S11.
Thiebaut A, Vernant JP, Degos L . Adult acute lymphocytic leukemia study testing chemotherapy and autologous and allogeneic transplantation: A follow-up report of the French protocol LALA 87. Hematol Oncol Clin North Am 2000; 14: 1353–1366.
Miano M, Labopin M, Hartmann O, Angelucci E, Cornish J, Gluckman E, et al., Paediatric Diseases Working Party of the European Group for Blood and Marrow Transplantation. Haematopoietic stem cell transplantation trends in children over the last three decades: a survey by the paediatric diseases working party of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant 2007; 39: 89–99.
Bunin N, Carston M, Wall D, Adams R, Casper J, Kamani N, et al., National Marrow Donor Program Working Group. Unrelated marrow transplantation for children with acute lymphoblastic leukemia in second remission. Blood 2002; 99: 3151–3157.
Woolfrey Ann E, Anasetti C, Storer B, Doney K, Milner LA, Sievers Eric L et al. Factors associated with outcome after unrelated marrow transplantation for treatment of acute lymphoblastic leukaemia in children. Blood 2002; 99: 2002–2008.
Kiehl MG, Kraut L, Schwerdtfeger R, Hertenstein B, Remberger M, Kroeger N et al. Outcome of allogenic hematopoietic stem-cell transplantation in adult patients with acute lymphoblastic leukemia: no difference in related compared with unrelated transplant in first complete remission. J Clin Oncol 2004; 22: 2816–2825.
Carlens S, Ringden O, Remberger M, Lonngvist B, Hägglund H, Klaesson S et al. Risk factors for chronic graft-versus-host disease after bone marrow transplantation: a retrospective single centre analysis. Bone Marrow Transplant 1998; 22: 755–761.
Zecca M, Prete A, Rondelli R, Lanino E, Balduzzi A, Messina C et al. Chronic graft-versus-host disease in children: incidence, risk factors, and impact on outcome. Blood 2002; 100: 1192–1200.
Ministry of Health Central Health Services Council. The Welfare of Children in Hospital: Report of the Committee. Ministry of Health Central Health Services Council: London, UK, 1959.
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
About this article
Cite this article
Dini, G., Banov, L., Dini, S. et al. Where should adolescents with ALL be treated?. Bone Marrow Transplant 42 (Suppl 2), S35–S39 (2008). https://doi.org/10.1038/bmt.2008.281
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2008.281