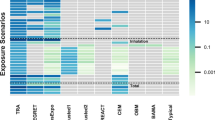
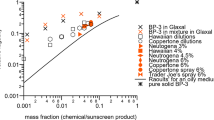
Abstract
Exposure scenarios form an essential basis for chemical risk assessment reports under the new EU chemicals regulation REACH (Registration, Evaluation, Authorisation and restriction of Chemicals). In case the dermal route of exposure is predominant, information on both exposure and dermal bioavailability is necessary for a proper risk assessment. Various methodologies exist to measure dermal exposure, providing quantitative or semiquantitative information. Although these studies may provide very specific and relevant information, it should be realized that case by case in-depth exposure assessment would be a very expensive process. Dermal bioavailability data are most often obtained from in vitro studies or animal experiments. For the design of studies, which generate data relevant for chemical risk assessment, detailed information on the exposure conditions is crucial (skin surface exposed, exposure duration, dose and physical state of the chemical). Results from non-testing methods for skin absorption, such as (Q)SARs, have been used only to a very limited extent for regulatory purposes. Suggestions are made in order to extend the use these methods to dermal risk assessment of chemical substances, thereby improving the practicability of REACH.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Bouwman T., Cronin M.T.D., Bessems J.G.M., and Van de Sandt J.J.M. Evaluation of published QSARs for percutaneous absorption. Toxicol Lett (2006): 164: S322.
Brouwer D.H., Aitken R.J., Oppl R., and Cherrie J.W. Concepts of skin protection: considerations for the evaluation and terminology of the performance of skin protective equipment. J Occup Environ Hyg (2005): 2: 425–434.
Buist H.E., de Heer C., Bessems J.G.M., Bouwman T., Chou S., Pohl H.R., and Van de Sandt J.J.M. Dermal absorption in risk assessment: the use of relative absorption versus permeation coefficient (Kp). Occupational and Environmental Exposures of Skin to Chemicals — 2005 (2005). Abstract for poster 25 http://www.cdc.gov/niosh/topics/skin/OEESC2/AbPost025Buist.html.
Buist H.E., de Heer C., van Burgsteden J.A., and van de Sandt J.J.M. Dermatokinetics of didecyldimethylammonium chloride and the influence of some commercial biocidal formulations on its dermal absorption in vitro. Regul Toxicol Pharmacol (2007) (E-pub ahead of print).
CEFIC. CEFIC Workshop on methods to determine dermal permeation for human risk assessment. Research Report TM/04/07 December 2004 (2004), 1–86. http://www.iom-world.org/news/ppworkshop.php.
Cherrie J.W., and Robertson A. Biologically relevant assessment of dermal exposure. Ann Occup Hyg (1995): 39: 387–392.
Chilcott R.P., Barai N., Beezer A.E., Brain S.I., Brown M.B., Bunge A.L., Burgess S.E., Cross S., Dalton C.H., Dias M., Farinha A., Finnin B.C., Gallagher S.J., Green D.M., Gunt H., Gwyther R.L., Heard C.M., Jarvis C.A., Kamiyama F., Kasting G.B., Ley E.E., Lim S.T., McNaughton G.S., Morris A., Nazemi M.H., Pellett M.A., DU Plessis J., Quan Y.S., Raghavan S.L., Roberts M., Romonchuk W., Roper C.S., Schenk D., Simonsen L., Simpson A., Traversa B.D., Trottet L., Watkinson A., Wilkinson S.C., Williams F.M., Yamamoto A., and Hadgraft J. Inter- and intralaboratory variation of in vitro diffusion cell measurements: an international multicenter study using quasi-standardized methods and materials. J Pharm Sci (2005): 94: 632–638.
Cleek R.L., and Bunge A.L. A new method for estimating dermal absorption from chemical exposure. 1. general approach. Pharm Res (1993): 10 (4): 497–506.
Cronin M.T.D. The prediction of skin permeability using quantitative structure-activity relationships (QSARs). In: Riviere J. (Ed.). Dermal Absorption Models in Toxicology and Pharmacology. CRC Press: Boca Raton, FL, USA, 2005.
De Heer C., Wilschut A., Stevenson H., and Hakkert B.C., Guidance document on the estimation of dermal absorption according to a tiered approach: an update. Zeist, Netherlands, TNO report V98.1237 (1999), 1–42. To be obtained through J.J.M. van de Sandt ( han.vandesandt@tno.nl).
EC. Technical guidance document (TGD) on risk assessment in support of Commission Directive 93/67/EEC on risk assessment for new notified substances. European Commission, Part, 1 (2003), 1–303.
EC. Guidance document on dermal absorption. European Commission, Sanco/222/2000 rev. 7 (2004), 1–15.
Feldman R.J., and Maibach H.I. Regional variation in percutaneous penetration of 14C cortisol in man. J Invest Dermatol (1967): 48: 181–183.
Fitzpatrick D., Corish J., and Hayes B. Modelling skin permeability for risk assessment — the future. Chemosphere (2004): 55: 1309–1314.
Flynn G.L. Physicochemical determinants of skin absorption. In: Gerrity T.R., Henry C.J. (Eds.). Principles of Route-to-Route Extrapolation for Risk Assessment. Elsevier: New York, 1990, pp 93–127.
Global Net on “Consumer Exposure Modelling”. Report of Workshop No. 1 on “Dermal transfer and penetration algorithms”. (2006). (Stylianos Kephalopoulos, Joop J. van Hemmen, Katinka van der Jagt). EUR 22521 EN/1 — DG Joint Research Centre, Institute for Health and Consumer Protection, Luxembourg: Office for Official Publications of the European Communities.
Hueber-Becker F., Nohynek G.J., Dufour E.K., Meuling W.J.A., de Bie AThHJ, Toutain H., and Bolt H.M. Occupational exposure of hairdressers to [(14)C]-para-phenylenediamine-containing oxidative hair dyes: a mass balance study. Food Chem Toxicol (2006) (E-pub ahead of print).
ICH (International conference on harmonization). Guideline for good clinical practise. ICH Harmonised Tripartite Guideline (1996), 1–57. http://www.ich.org.
IPCS. International Programme on Chemical Safety, Environmental Health Criteria 235: dermal absorption. United Nations Environment Programme, World Health Organisation (2006), WHO Press: Geneva, Switzerland.
Jakasa Y., Mohammadi N., Kruse J., and Kezic S. Percutaneous absorption of neat and aqueous solutions of 2-butoxyethanol in volunteers. Int Arch Occup Environ Health (2004): 77: 79–84.
Kroes R., Renwick A.G., Feron V., Galli C.L., Gibney M., Greim H., Guy R.H., Lhuguenot J.C., and Van de Sandt J. Application of the threshold of toxicological concern (TTC) to the safety evaluation of cosmetic ingredients, (2007) (submitted).
Kromhout H., Fransman W., Vermeulen R., Roff M., and Van Hemmen J.J. Variability of task-based dermal exposure measurements from a variety of workplaces. Ann Occup Hyg (2004): 48: 187–196.
Kruse J., Golden D., Wilkinson S., Williams F., Kezic S., and Corish J. Analysis, interpretation, and extrapolation of dermal permeation data using diffusion-based mathematical models. Pharm Sci (2007): 96: 682–703.
Moss G.P., Dearden J.C., Patel H., and Cronin M.D. Quantitative structure-permeability relationships (QSPRs) for percutaneous absorption. Toxicol In Vitro (2002): 16: 299–317.
OECD. OECD guideline for the testing of chemicals. Skin Absorption: In Vivo Method (2004a): 427. Adopted: 13 April 2004, pp 1–8, OECD publishing, Paris.
OECD. OECD guideline for the testing of chemicals. Skin Absorption: In Vitro Method (2004b): 428. Adopted 13 April 2004, pp 1–8, OECD publishing, Paris.
OECD. Guidance document for the conduct of skin absorption studies. OECD Series on Testing and Assessment (2004c) Number 28 ENV/JM/MONO (2004), pp 21–31, OECD publishing, Paris.
Potts R.O., and Guy R.H. Predicting skin permeability. Pharm Res (1992): 9: 663–669.
RISKOFDERM. Special Issue. Ann Occup Hyg (2004): 48: 183–297.
Ross J.S., and Shah J.C. Reduction in skin permeation of N,N-diethyl-m-toluamide (DEET) by altering the skin/vehicle partition coefficient. J Control Release (2000): 67: 211–221.
SCCP (Scientific Committee on Consumer Products). Basic criteria for the in vitro assessment of dermal absorption of cosmetic ingredients. Document SCCP/0790/06 Adopted by the SCCP on March 28 (2006).
Scheuplein R.J., and Blank I.H. Permeability of the skin. Physiol Rev (1971): 51: 702–747.
Southwell J.D., Barry B.W., and Woodford R. Variations in permeability of human skin within and between specimens. Int J Pharm (1984): 18: 299–309.
Steiling W., Kreutz J., and Hofer H. Percutaneous penetration/dermal absorption of hair dyes in vitro. Toxicology In Vitro (2001): 15: 565–570.
Touraille G.D., McCarley K.D., Bunge A.L., Marty J.P., and Guy R.H. Percutaneous absorption of 4-cyanophenol from freshly contaminated soil in vitro: effects of soil loading and contamination concentration. Environ Sci Technol (2005): 39: 3723–3731.
US EPA. Dermal exposure assessment: principles and applications. EPA/600/8-91/011B (1992), 1–389. http://www.epa.gov/nceawww1/pdfs/derexp.pdf.
US EPA. Health effects test guidelines. US Environmental Protection Agency, OPPTS 870.7600. Dermal penetration (1998), 1–12. http://www.epa.gov/opptsfrs/OPPTSHarmonized/870HealthEffectsTestGuidelines/Series/870-7600.pdf.
US EPA. In vitro dermal absorption rate testing of certain chemicals of interest to the occupational safety and health administration; Final rule. Fed Regist (2004a): 69 (80): 22402–22441.
US EPA. Risk assessment guidance for superfund volume I: human health evaluation manual (part E, supplemental guidance for dermal risk assessment). EPA540/R/99/005 (2004b), 1–156. http://www.epa.gov/oswer/riskassessment/ragse/pdf/20041101parte.pdf.
Van de Sandt J.J.M., van Burgsteden J.A., Carmichael P.L., Dick I., Kenyon S., Korinth G., Larese F., Limasset J.C., Maas W.J.M., Montomoli L., Nielsen J.B., Payan J.-P., Robinson E., Sartorelli P., Schaller K.H., Wilkinson S.C., and Williams F.M. In vitro predictions of skin absorption of caffeine, testosterone, and benzoic acid: a multi-centre comparison study. Regul Toxicol Pharmacol (2004): 39: 271–281.
Van Hemmen J.J., and Brouwer D.H. Assessment of dermal exposure to chemicals. Sci Total Environm (1995): 168: 131–141.
Van Hemmen J.J., and Van der Jagt K.E. Generic Operator exposure databases. In: Franklin C.A., Worgan J.P. (Eds.). Occupational and Residential Exposure Assessment for Pesticides. Wiley and Sons: UK, 2005.
Van Ravenzwaay B., and Leibold E. The significance of in vitro rat skin absorption studies to human risk assessment. Toxicology In Vitro (2004): 18: 219–225.
Walker J.D., Whittaker C., and McDougal J.N. Role of the TSCA Interagency Testing Committee in Meeting the US Government's Data Needs: Designating chemicals for percutaneous absorption testing. In: Marzulli F.N., Maibach H.I. (Eds.). Dermatotoxicology, 5th edn Hemisphere Publishing Corporation: Washington, DC, (1996), 371–381.
Wester R.C., and Maibach H.I. Regional variation in percutaneous absorption. In: Bronaugh R.L., Maibach H.I. (Eds.). Percutaneous Absorption: Drugs—Cosmetics — Mechanism — Methodology. (3rd ed., rev. and expanded). [Drugs and pharmaceutical science Vol. 97] Marcel Dekker Inc: New York, (1999), pp 107–116.
Wheeler J.P., and Warren N.D. A Dirichlet Tesselation-based sampling scheme for measuring whole-body exposure. Ann Occup Hyg (2002): 46: 209–217.
WMO (World Medical Association). Declaration of Helsinki (Ethical principles for medical research involving human subjects) (2004), 1–5. http://www.wma.net.
Zartarian V., Bahador T., and McKone T. Adoption of an official ISEA glossary. J Expo Anal Environ Epidemiol (2005): 15: 1–15.
Acknowledgements
This paper addresses various approaches on dermal exposure assessment used at present in regulatory risk assessment and identifies some aspects needing improvement. The text and recommendations are partly based on the discussions and proceedings of the Workshop “Quantitative Risk Assessment” (OEESC-2005 Conference, Stockholm, Sweden) and the workshop “Dermal Transfer and Penetration Algorithms” (Global Net on Consumer Exposure Modeling, June 20/21, 2005, Verbania/Intra (Italy)) in which, in addition to the authors, the following individuals participated (Global Net on “Consumer Exposure Modelling”: Report of Workshop No. 1 on “Dermal transfer and penetration algorithms”, 2006): ten Berge, Wil, Santoxar, Westervoort, The Netherlands; Bunge, Annette L., Colorado School of Mines, Golden, CO, USA; Corish, John, University of Dublin, Dublin, Ireland; Cronin, Mark, Liverpool John Moores University, Liverpool, England; Diembeck, Walter, Beiersdorf A.G., Hamburg, Germany; de Heer, Cees, TNO Quality of Life, Zeist, The Netherlands (now: RIVM, Bilthoven, The Netherlands); van der Jagt, Katinka E., EMEA, London, England (now: DG Enterprise EU, Brussels, Belgium); Kephalopoulos, Stelios, JRC/IHCP/PCE, Ispra, Italy; Kissel, John, University of Washington, Seattle, WA, USA; McCready, David, The Dow Chemical Company, South Charleston, West Virginia, USA; Papamelitiou, Demosthenos, JRC, IHCP, PCE, Ispra, Italy; Patlewiecz, Grace, JRC/IHCP/ECB, Ispra, Italy; Perkins, John, Dow Agroscience Ltd., Milton Park Abingdon, England; Ross, John, Infoscientific, Carmichael, CA, USA; Steiling, Winfried, Henkel KGaA, Düsseldorf, Germany; Tyler, Tip, Health Studies Management & Consulting, Annandale, VA, USA; Warren, Nick, Health and Safety Laboratory, Buxton, England; Williams, Faith, University of Newcastle, Newcastle, England and Worth, Andrew, JRC/IHCP/ECB, Ispra, Italy.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Van de Sandt, J., Dellarco, M. & Van Hemmen, J. From dermal exposure to internal dose. J Expo Sci Environ Epidemiol 17 (Suppl 1), S38–S47 (2007). https://doi.org/10.1038/sj.jes.7500579
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.jes.7500579
Keywords
This article is cited by
-
Dermal bioaccessibility of flame retardants from indoor dust and the influence of topically applied cosmetics
Journal of Exposure Science & Environmental Epidemiology (2017)
-
A framework incorporating the impact of exposure scenarios and application conditions on risk assessment of chemicals applied to skin
In Silico Pharmacology (2013)
-
Exposure and health risk assessment of applicators to DDT during indoor residual spraying in malaria vector control program
Journal of Exposure Science & Environmental Epidemiology (2012)
-
For good measure: Origins and prospects of exposure science (2007 Wesolowski Award Lecture)
Journal of Exposure Science & Environmental Epidemiology (2010)
-
Issues in consumer exposure modeling: Towards harmonization on a global scale
Journal of Exposure Science & Environmental Epidemiology (2007)