Abstract
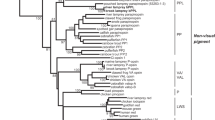
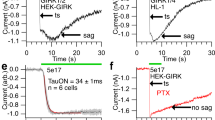
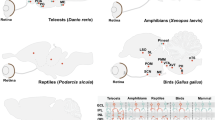
IN avian pinealocytes, an environmental light signal resets the phase of the endogenous circadian pacemaker that controls the rhythmic production of melatonin1–6. Investigation of the pineal phototransduction pathway should therefore reveal the molecular mechanism of the biological clock. The presence of rhodopsin-Iike photoreceptive pigment4,5 7–9, transducin-like immunoreaction10, and cyclic GMP-dependent cation-channel activity11 in the avian pinealocytes suggests that there is a similarity between retinal rod cells and pinealocytes in the phototransduction pathway. We have now cloned chicken pineal cDNA encoding the photoreceptive molecule, which is 43–48% identical in amino-acid sequence to vertebrate retinal opsins. Pineal opsin, produced by transfection of complementary DNA into cultured cells, was reconstituted with 11-cis-retinal, resulting in formation of a blue-sensitive pigment λmax ≈470 nm). In the light of this functional evidence and because the gene is specifically expressed only in the pineal gland, we con-clude that it is a pineal photosensor and name it pinopsin.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Binkley, S. A., Riebman, J. B. & Reilly, K. B. Science 202, 1198–1201 (1978).
Kasal, C. A., Menaker, M. & Prez-Polo, J. R. Science 203, 656–658 (1979).
Deguchi, T. Science 203, 1245–1247 (1979).
Deguchi, T. Nature 290, 706–707 (1981).
Wallingford, J. C. & Zats, M. Exp. Eye Res. 46, 909–918 (1988).
Takahashi, J. S., Murakami, N., Nikaido, S. S., Pratt, B. L. & Robertson, L. M. Rec. Progr. Horm. Res. 45, 279–352 (1989).
Foster, R. G., Schalken, J. J., Timmers, A. M. & DeGrip, W. J. J. comp. Physiol. A 165, 553–563 (1989).
Araki, M., Fukada, Y., Shichida, Y., Yoshizawa, T. & Tokunaga, F. Devl Brain Res. 65, 85–92 (1992).
Masuda, H. et al. Tiss. Cell 26, 101–113 (1994).
van Veen, T. et al. Proc. natn. Acad. Sci. U.S.A. 83, 912–916 (1986).
Dryer, S. E. & Henderson, D. Nature 353, 756–758 (1991).
Kuwata, O. et al. FEBS Lett. 272, 128–132 (1990).
Okano, T., Kojima, D., Fukada, Y., Shichida, Y. & Yoshizawa, T. Proc. natn. Acad. Sci. U.S.A. 89, 5932–5936 (1992).
Saitou, N. & Nei, M. Molec. Biol. Evol. 4, 406–425 (1987).
Kojima, D. et al. Proc. natn. Acad. Sci. U.S.A. 89, 6941–6845 (1992).
Sun, J.-H., Reiter, R. J., Mata, N. L. & Tsin, A. T. C. Neurosci. Lett. 133, 97–99 (1991).
Okano, T., Fukada, Y., Artamonov, I. D. & Yoshizawa, T. Biochemistry 28, 8848–8856 (1989).
Zats, M. & Mullen, D. A. Brain Res. 453, 63–71 (1988).
Takao, M., Yasui, A. & Tokunaga, F. Vision Res. 28, 471–480 (1988).
Sambrook, J., Fritsch, E. F. & Maniatis, T. Molecular Cloning: A Laboratory Manual 2nd edn (Cold Spring Harbor Laboratory Press, New York, 1989).
Nathans, J. Biochemistry 29, 9746–9752 (1990).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Okano, T., Yoshizawa, T. & Fukada, Y. Pinopsin is a chicken pineal photoreceptive molecule. Nature 372, 94–97 (1994). https://doi.org/10.1038/372094a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/372094a0
This article is cited by
-
The non-visual opsins expressed in deep brain neurons projecting to the retina in lampreys
Scientific Reports (2020)
-
Evolutionary history of teleost intron-containing and intron-less rhodopsin genes
Scientific Reports (2019)
-
Brain-specific homeobox Bsx specifies identity of pineal gland between serially homologous photoreceptive organs in zebrafish
Communications Biology (2019)
-
Pinopsin evolved as the ancestral dim-light visual opsin in vertebrates
Communications Biology (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.