Abstract
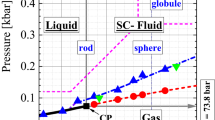
ABOVE a substance's liquid–vapour critical point (Tc), the distinction between the liquid and vapour phases disappears. Below the triple point (Tt), meanwhile (at which solid, liquid and vapour coexist), only the solid and vapour are stable. The liquid range, Tc/Tt, depends on the nature of the intermolecular forces: for argon, Tc/Tt = 1.8, whereas for sodium the ratio is 7.5. But might there be molecular substances that have no liquid phase at all? Here we present results which suggest that C60 is such a substance. We map out the phase diagram using computer simulations in which the C60 molecules are represented by spheres interacting via Lennard-Jones potentials summed over all 60 carbon atoms. We find that the sublimation line passes above the metastable liquid-vapour coexistence curve. By drawing an analogy with the aggregation of colloidal particles, we expect that solid C60 formed by nucleation from the vapour phase will be amorphous rather than crystalline.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Girifalco, L. A. J. phys. Chem. 96, 858–861 (1992).
Pusey, P. N. in Liquids, Freezing and Glass Transition (eds Hansen, J. P., Levesque, D. & Zinn-Justin J.) 763ndash;942 (North-Holland, Amsterdam, 1991).
Heiney, P. A. et al. Phys. Rev. Lett. 66, 2911–2914 (1991).
Nunez-Regueiro, M. Mod. Phys. Lett. B19, 1153–1158 (1992).
Panagiotopoulos, A. Z. Molec. Phys. 61, 813–826 (1987).
Smit, B., de Smedt, Ph. & Frenkel, D. Molec. Phys. 68, 931–950 (1989).
Frenkel, D. & Ladd, A. J. C. J. chem. Phys. 81, 3188–3193 (1984).
Meijer, E. J., Frenkel, D., LeSar, R. A. & Ladd, A. J. C. J. chem. Phys. 92, 7570–7575 (1990).
Kofke, D. A. J. chem. Phys. 98, 4149–4162 (1993).
Rouw, P. W. & de Kruif, C. G. Phys. Rev. A39, 5399–5408 (1989).
Kranendonk, W. G. T. & Frenkel, D. Molec. Phys. 64, 403–424 (1988).
Cheng, A., Klein, M. L. & Caccamo, C. Phys. Rev. Lett. 71, 1200–1203 (1993).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hagen, M., Meijer, E., Mooij, G. et al. Does C60 have a liquid phase?. Nature 365, 425–426 (1993). https://doi.org/10.1038/365425a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/365425a0
This article is cited by
-
Intermolecular Potential Model Hamiltonians for Gas–Liquid Coexistence
International Journal of Thermophysics (2022)
-
Assembly and phase transitions of colloidal crystals
Nature Reviews Materials (2016)
-
Unravelling the multilayer growth of the fullerene C60 in real time
Nature Communications (2014)
-
Dynamic lattice searching methods for optimization of clusters
Frontiers of Chemistry in China (2009)
-
The role of interparticle and external forces in nanoparticle assembly
Nature Materials (2008)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.