Abstract
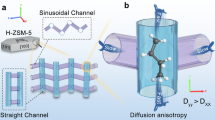
THE catalytic conversion of methanol to hydrocarbons in the gasoline boiling range1 (30–200 °C) using zeolite ZSM-5 at ∼370 °C has attracted much attention and is now used industrially. Methanol (CH3OH) is dehydrated to dimethyl ether (DME) and the equilibrium mixture of CH3OH and DME is then converted to olefins, aliphatics and aromatics up to C10. However, the mechanism of the reactions involved, particularly in regard to the formation of the first carbon–carbon bond and the nature of the intermediates, remains a matter for speculation. Here we report the use of 13C magic-angle-spinning NMR to identify 29 different organic species in the adsorbed phase, and to monitor their fate during the course of the reaction. This technique allows us to observe different kinds of shape selectivity in the zeolite host, to identify CO as an intermediate in the reaction, and to distinguish unequivocally between mobile and attached species. The results will assist the design of shape-selective solids and provide a better understanding of catalytic processes in the intracrystalline space.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Meisel, S. L., McCullough, J. P., Lechthaler, C. H. & Weisz, P. B. Chemtech 6, 86–89 (1976).
Weisz, P. B. & Frilette, V. J. J. phys. Chem. 64, 382 (1960).
Csicsery, S. M. in Zeolite Chemistry and Catalysis (ed. Rabo, J. A.), ACS Monograph 171, 680–713 (1976).
Chen, N. Y., Kaeding, W. W. & Dwyer, F. G. J. Am. chem. Soc. 101, 6783–6784 (1979).
Kaeding, W. W. US Patent No. 4,029,716 (1977).
Haag, W. O. & Olson, D. H. US Patent No. 4,097,543 (1978).
Carpenter, T. A., Klinowski, J., Tennakoon, D. T. B., Smith, C. J. & Edwards, D. C. J. magn. Reson. 68, 561–563 (1986).
Derouane, E. G. et al. J. Catal. 53, 40–55 (1978).
Derouane, E. G. et al. C.r. Acad Sci. Paris Ser. C 284, 945–948 (1977).
Nagy, J. B., Gilson, J. P. & Derouane, E. G. J. molec. Catal. 5, 393–397 (1979).
Derouane, E. G., Dejaifve, P. & Nagy, J. B. J. molec. Catal. 3, 453–457 (1977).
Derouane, E. G. & Nagy, J. B. ACS Symp. Ser. 248, 101–125 (1984).
Derouane, E. G., Gilson, J. P. & Nagy, J. B. Zeolites 2, 42–46 (1982).
Bronnimann, C. E. & Maciel, G. E. J. Am. chem. Soc. 108, 7154–7159 (1986).
Stothers, J. B. Carbon-13 NMR Spectroscopy (Academic, New York, 1972).
Denney, D., Mastikhin, V. M., Namba, S. & Turkevich, J. J. phys. Chem. 82, 1752–1760 (1978).
Meier, W. M. & Olson, D. H. Atlas of Zeolite Structure Types (Butterwoths, Kent, 1988).
Chang, C. D. Catal. Rev.-Sci. Eng. 25, 1–118 (1983).
Chang, C. D., Lang, W. H. & Bell, W. K. Catalysis of Organic Reactions (ed. Moser, W. R.) 73–94 (Dekker, New York, 1981).
Chang, C. D. & Silvestri, A. J. J. Catal. 47, 249–259 (1977).
Hastings, S. H. & Nicholson, D. E. J. Chem. Eng. Data 6, 1–4 (1961).
Lyerla, J. R., Yannoni, C. S. & Fyfe, C. A. Acct. chem. Res. 15, 201–208 (1982).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Anderson, M., Klinowski, J. Direct observation of shape selectivity in zeolite ZSM-5 by magic-angle-spinning NMR. Nature 339, 200–203 (1989). https://doi.org/10.1038/339200a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/339200a0
This article is cited by
-
Computational prediction of the molecular configuration of three-dimensional network polymers
Nature Materials (2021)
-
One-dimensional intergrowths in two-dimensional zeolite nanosheets and their effect on ultra-selective transport
Nature Materials (2020)
-
The Effect of Co-feeding Methyl Acetate on the H-ZSM5 Catalysed Methanol-to-Hydrocarbons Reaction
Topics in Catalysis (2020)
-
Microwaves effectively examine the extent and type of coking over acid zeolite catalysts
Nature Communications (2017)
-
In situ 13C MAS NMR Study on the Mechanism of Butane Isomerization Over Catalysts with Different Acid Strength
Topics in Catalysis (2005)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.