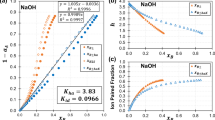
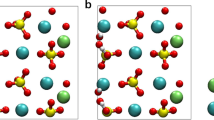
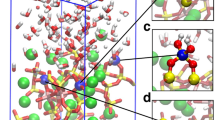
Abstract
Surface species control the mechanism and rate of low-temperature silicate dissolution and precipitation reactions in dilute solutions. This has been repeatedly emphasized1–;7. The interaction between dissolved species and silicate surfaces involves exchange and adsorption phenomena, which equilibrate rapidly, and are accessible to determination by surface titration8,9. Here we report a comparison of surface species concentrations with the dissolution rates of olivine and albite which indicates that while the dissolution rates are a complex function of solution pH, the dissolution rates have a simple first-order dependence on surface concentrations of specific surface species. Thus, the interpretation of the kinetic data for olivine and albite dissolution becomes simple when the chemical speciation at the mineral surface is incorporated, and this approach holds great promise for unifying the rate data for many important silicates.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Stumm, W., Furrer, G., Wieland, E. & Zinder, B. in The Chemistry of Weathering (ed. Drever, J. I.) 55–74 (Reidel, Dordrecht, 1985).
Helgeson, H. C., Murphy, W. M. & Aagard, P. Geochim. cosmochim. Acta 48, 2405–2432 (1984).
Lasaga, A. C. in Kinetics of Geochemical Processes (eds Lasaga, A. C. & Kirkpatrick, R. J.) 35–48 (Mineral. Soc. Am., Washington DC, 1981).
Rimstidt, J. D. & Dove, P. M. Geochim. cosmochim. Acta 50, 2509–2516 (1986).
Chou, L. & Wollast, R. Am. J. Sci. 285, 963–993 (1985).
Holdren, G. R. & Speyer, P. M. Am. J. Sci. 285, 994–1026 (1985).
Tole, M. P., Lasaga, A. C., Pantano, C. & White, W. B. Geochim. cosmochim. Acta 50, 379–392 (1986).
Stumm, W. & Morgan, J. J. Aquatic Chemistry (Wiley, New York, 1981).
Haung, C. P. in Adsorption of Inorganics at Solid - Liquid Interfaces (eds Anderson, M. A. & Rubin, A. J.) 183–218 (Ann Arbor Sci., Ann Arbor, Michigan, 1981).
Berner, R. A. & Holdren, G. R. Geochim. cosmochim. Acta 43, 1173–1186 (1979).
Chou, L. & Wollast, R. Geochim. cosmochim. Acta 48, 2205–2217v (1984).
Chou, L. & Wollast, R. in The Chemistry of Weathering (ed. Drever, J. I.) 75–96 (Reidel, Dordrecht, 1985).
Holdren, G. R. & Speyer, P. M. Geochim. cosmochim. Acta 49, 675–681 (1985).
Grandstaff, D. E. Geochim. cosmochim. Acta 42, 1899–1901 (1978).
Grandstaff, D. E. in Rates of Chemical Weathering (eds Colman, S. M. & Dethier, D.) 41–59 (Academic, New York, 1986).
Lasaga, A. C. J. geophys. Res. 89, 4009–4025 (1984).
Helgeson H. C. Nature 325, 667 (1987).
Petrovic, R., Berner, R. A. & Goldhaber, M. B. Geochim. cosmochim. Acta 40, 537–548 (1976).
Fung, P. C., Bird, G. W., Mclntyre, G. G., Sanipelli, G. G. & Lopata, V. J. Nucl. Tech. 51, 188–196 (1980).
Schott, J. & Berner, R. A. Geochim. cosmochim. Acta 47, 2233–2240 (1983).
Hochella, M. F., Ponader, H. B., Turner, A. M. & Harris, D. W. Geochim. cosmochim. Acta (in the press).
Petit, J., Mea, G. D., Dran, J., Schott, S. & Berner, R. A. Nature 325, 705–707 (1987).
Helgeson, H. C. Geochim. cosmochim Acta 35, 421–469 (1971).
Helgeson, H. C. Geochim. cosmochim. Acta 36, 1067–1070 (1972).
Furrer, G. & Stumm, W. Geochim. cosmochim. Acta 50, 1847–1860 (1986).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Blum, A., Lasaga, A. Role of surface speciation in the low-temperature dissolution of minerals. Nature 331, 431–433 (1988). https://doi.org/10.1038/331431a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/331431a0
This article is cited by
-
Sustainable next-generation single-component geopolymer binders: a review of mechano-chemical behaviour and life-cycle cost analysis
Journal of Material Cycles and Waste Management (2024)
-
Crystal dissolution by particle detachment
Nature Communications (2023)
-
New estimate of chemical weathering rate in Xijiang River Basin based on multi-model
Scientific Reports (2021)
-
Carbon dioxide storage through mineral carbonation
Nature Reviews Earth & Environment (2020)
-
Rocks control the chemical composition of surface water from the high Alpine Zermatt area (Swiss Alps)
Swiss Journal of Geosciences (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.