Abstract
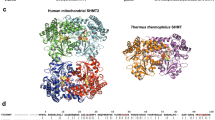
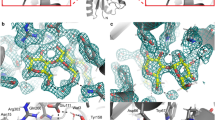
An important question in understanding substrate binding by proteins is how charged groups are stabilized in the absence of their solvation shell. We have addressed this question here by solving the structure of the sulphate-binding protein of Salmonella typhimurium with bound substrate at 2.0 Å resolution. The results are remarkable in that the charged oxygen atoms of the sulphate molecule, which is buried and completely inaccessible to the solvent, are not stabilized by the formation of salt-bridges but by hydrogen bonds donated by specific residues of the protein. These hydrogen bonds are in turn coupled via peptide units to several resonating hydrogen bonding systems. These findings may be of general significance for the role of electrostatic interactions in protein structure and function.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Quiocho, F. A. & Vyas, N. K. Nature 310, 381–386 (1984).
Vyas, N. K., Vyas, M. N. & Quiocho, F. A. Proc. natn. Acad. Sci. U.S.A. 80, 1792–1796 (1983).
Saper, M. A. & Quiocho, F. A. J. biol. Chem. 258, 11057–11062 (1983).
Landick, R. & Oxender, D. L. in Bacterial Transport (ed. Martonosi, A. N.) 81–88 (Plenum, New York, 1982).
Hendrickson, W. A. & Konnert, J. H. in Computing in Crystallography (eds Diamond, R., Ramaseshan, S. & Venkatesan, K.) 13.01–13.23 (Indian Academy of Sciences, International Union of Crystallography, Bangalore, 1980).
Lee, B. K. & Richards, F. M. J. molec. Biol. 55, 517–526 (1971).
Pauling, L. The Nature of the Chemical Bond, 325 (Cornell University Press, Ithaca, 1966).
Hol, W. G. J., van Duijnen, P. T. & Berendsen, H. J. C. Nature 273, 443–446 (1978).
Rees, D. C. J. molec. Biol. 141, 323–326 (1980).
Perutz, M. F., Kendrew, J. C. & Watson, H. C. J. molec. Biol. 13, 669–678 (1965).
Blundell, T., Barlow, D., Borkakoti, N. & Thornton, J. Nature 306, 281–283 (1983).
Smith, W. W., Burnett, R. M., Darling, G. D. & Ludwig, M. L. J. molec. Biol. 117, 195–225 (1977).
Bernstein, F. C. et al. J. molec. Biol. 112, 535–542 (1977).
Miller, D. M. III, Olson, J. S., Pflugrath, J. W. & Quiocho, F. A. J. biol. Chem. 258, 13665–13672 (1983).
Isihara, H. & Hogg, R. W. J. biol Chem. 255, 4614–4618 (1980).
Lesk, A. M. & Hardman, K. D. Science 216, 539–540 (1982).
Jones, T. A. in Computational Crystallography (ed. Sayre, D.) 3303–3317 (Clarendon, Oxford, 1982).
Pflugrath, J. W., Saper, M. A. & Quiocho, F. A. in Methods and Applications in Crystallographic Computing (eds Hall, S. & Ashida, T.) 404–407 (Clarendon, Oxford, 1984).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Pflugrath, J., Quiocho, F. Sulphate sequestered in the sulphate-binding protein of Salmonella typhimurium is bound solely by hydrogen bonds. Nature 314, 257–260 (1985). https://doi.org/10.1038/314257a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/314257a0
This article is cited by
-
Solvent effects in anion recognition
Nature Reviews Chemistry (2024)
-
Clever cryptand cage coordinates contaminants
Nature Chemistry (2024)
-
Rigid macrocycles with multiple hydrogen-bond donors for effective anion binding and transport
Nature Chemistry (2023)
-
Aromatic pentaamide macrocycles bind anions with high affinity for transport across biomembranes
Nature Chemistry (2023)
-
Are beryllium-containing biphenyl derivatives efficient anion sponges?
Journal of Molecular Modeling (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.