Abstract
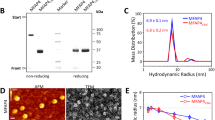
Fibronectins are adhesive glycoproteins thought to mediate the attachment of cells to various substrates1,2. Plasma fibronectin (PFN) is a dimer comprising subunits of molecular weight 220,000, connected by one or two disulphide bonds. Electron microscopy shows that PFN is a long, flexible strand, 2–3 nm in diameter and 140 nm long3,4. Many cells in tissue culture elaborate an extracellular matrix of insoluble (highly cross-linked by disulphide bonds) fibronectin, and a variable amount of ‘cell surface fibronectin’ (CSFN) that can be extracted by mild urea treatment. This CSFN, soluble in 1 M urea and at high pH, is a mixture of dimers and disulphide-bonded oligomers5–7. In the present study we have examined the structure of these molecules by electron microscopy. Oligomers were separated from dimers and contaminating proteins by zone sedimentation through glycerol gradients. We report that the CSFN dimers are identical in structure to PFN. In contrast, the oligomers have an elaborate and well defined structure that we call a ‘hexabrachion’: six arms emanating from a central globular particle. The arms are similar to PFN in being long, thin and flexible, but have several distinctly different features.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Yamada, K. M. A. Rev. Biochem. 52, 761–797 (1983).
Mosher, D. Prog. Hemostasis Thrombosis 5, 111–150 (1980).
Engel, J. et al. J. molec. Biol. 150, 97–120 (1981).
Erickson, H. P., Carrell, N. A. & McDonagh, J. J. Cell Biol. 91, 673–678 (1981).
Yamada, K. M., Schlesinger, D. H., Kennedy, D. W. & Pastan, I. Biochemistry 16, 5552–5559 (1977).
Hynes, R. O. & Destree, A. Proc. natn. Acad. Sci. U.S.A. 74, 2855–2859 (1977).
Alexander, S. S., Colonna, G., Yamada, K. M., Pastan, I. & Edelhoch, H. J. biol. Chem. 253, 5820–5824 (1978).
Yamada, K. M. & Kennedy, D. W. J. Cell Biol. 80, 492–498 (1979).
Erickson, H. P. & Carrell, N. A. J. biol. Chem. 258, 14539–14544 (1983).
Iglesias, J. L. & Erickson, H. P. J. Cell Biol. 97, 1233a (1983).
Chiquet, M. & Fambrough, D. M. J. Cell Biol. 98, 1926–1936, 1937–1948 (1984).
Laemmli, U. K. Nature 227, 600–685 (1970).
Oakley, B. R., Kirsch, D. R. & Morris, N. R. Analyt. Biochem. 105, 361–363 (1980).
Fowler, W. E. & Erickson, H. P. J. molec. Biol. 134, 241–249 (1979).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Erickson, H., Inglesias, J. A six-armed oligomer isolated from cell surface fibronectin preparations. Nature 311, 267–269 (1984). https://doi.org/10.1038/311267a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/311267a0
This article is cited by
-
Expression of tenascin-C and its isoforms in the breast
Cancer and Metastasis Reviews (2010)
-
A comparative analysis of oncofetal fibronectin and tenascin-C incorporation in tumour vessels using human recombinant SIP format antibodies
Histochemistry and Cell Biology (2010)
-
Size and Shape of Protein Molecules at the Nanometer Level Determined by Sedimentation, Gel Filtration, and Electron Microscopy
Biological Procedures Online (2009)
-
B and C domain containing tenascin-C: urinary markers for invasiveness of urothelial carcinoma of the urinary bladder?
Journal of Cancer Research and Clinical Oncology (2009)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.