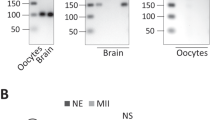
Abstract
A characteristic of growing oocytes of all animal species is the synthesis and accumulation of messenger RNA which is destined to be used primarily by the early embryo1,2. The mechanism(s) which regulates the translation of this maternal mRNA remains unknown. However, the inability of the oocyte to translate all of its putative mRNA has been attributed to at least three limitations: (1) The rate of translation is limited by the availability of components of the translational apparatus other than mRNA3,4, (2) the structural organization of the mRNA prevents translation5,6, and (3) proteins associated with the mRNA prevent translation7,8. Several investigators have suggested that proteins associated with maternal mRNA suppress translation in sea urchin eggs7–10, although others claim that such results may be due to experimental artefacts11,12. Oocyte-specific proteins have been identified in association with non-translating poly(A)+ mRNAs from Xenopus laevis oocytes13–16, and we report here that when these proteins are reconstituted with mRNAs in vitro the translation of the mRNAs in vitro is reversibly repressed. The implication is that these proteins are involved in the regulation of translation of stored maternal mRNAs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Davidson, E. H. Gene Activity in Early Development (Academic, New York, 1976).
Smith, L. D. & Richter, J. D. in Fertilization (eds Monroy, A. & Metz, C.) (in the press).
Laskey, R. A. Mills, A. D., Gurdon, J. B. & Partington, G. A. Cell 11, 345–351 (1977).
Richter, J. D. & Smith, L. D. Cell 27, 183–191 (1981).
Anderson, D. M. et al. J. molec. Biol. 155, 181–309 (1982).
Richter, J. D., Anderson, D. M., Davidson, E. H. & Smith, L. D. J. molec. Biol. 173, 227–242 (1984).
Ilan, J. & Ilan, J. Devl Biol. 66, 375–385 (1978).
Jenkins, N. A., Kaumeyer, J. F., Young, E. M. & Raff, R. A. Devl Biol. 63, 279–298 (1978).
Maundrell, K. et al. Molec. biol. Rep., 43–52 (1979).
Bergmann, I. E., Cereghini, S., Geohegan, I. & Brawerman, G. J. molec. Biol. 156, 567–582 (1982).
Moon, R. T., Danilchik, M. V. & Hille, M. B. Devl Biol. 93, 389–403 (1982).
Moon, R. T. Differentiation 24, 13–23 (1983).
Darnbrough, C. H. & Foord, P. J. Devl Biol. 50, 285–301 (1976).
Darnbrough, C. H. & Ford, P. J. Eur. J. Biochem. 113, 415–424 (1981).
Dixon, L. & Ford, P. J. Devl Biol. 93, 478–497 (1982).
Richter, J. D. & Smith, L. D. J. biol. Chem. 258, 4864–4869 (1983).
Pullman, J. M. & Martin, T. E. J. Cell Biol. 97, 99–111 (1983).
Economidis, I. V. & Pederson, T. Proc. natn. Acad. Sci. U.S.A. 80, 4296–4300 (1983).
Baer, B. W. & Kornberg, R. D. J. Cell Biol. 96, 717–721 (1983).
Spirin, A. S. Eur. J. Biochem. 10, 20–35 (1969).
Wasserman, W. J., Richter, J. D. & Smith, L. D. Devl Biol. 89, 152–158 (1982).
Richter, J. D., Wasserman, W. J. & Smith, L. D. Devl Biol. 89, 159–167 (1982).
Richter, J. D. & Evers, D. C. J. biol. Chem. 259, 2190–2194 (1984).
Wallace, R. A., Jared, D. W., Dumont, J. N. & Sega, M. W. J. exp. Zool. 18, 321–334 (1974).
Maniatis, T., Fritsch, E. F. & Sambrook, J. Molecular Cloning (Cold Spring Harbor Laboratory, New York, 1982).
Richter, J. D., Jones, N. C. & Smith, L. D. Proc. natn. Acad. Sci. U.S.A. 79, 3789–3793 (1983).
Spirin, A. S., Belitsina, N. V. & Lerman, M. I. J. molec. Biol. 14, 611–615 (1965).
Kloetzel, P.-M., Whitfield, W. & Sommerville, J. Nucleic Acids Res. 9, 605–621 (1981).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Richter, J., Smith, L. Reversible inhibition of translation by Xenopus oocyte-specific proteins. Nature 309, 378–380 (1984). https://doi.org/10.1038/309378a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/309378a0
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.