Abstract
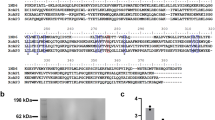
Tonin1, an ester protease isolated from rat sub maxillary gland, is a serine protease with trypsin-and chymotrypsin-like activity. The substrate specificity of tonin shows that it differs from kallikreins and is definitely not a renin-like enzyme or an angiotensin-converting enzyme2. Tonin can produce directly the vasoactive peptide angiotensin II, from angiotensin I, angiotensinogen and the synthetic tetradecapeptide substrate of renin by cleavage of a Phe-His bond. It has also been found to cleave some Phe and Arg bonds in various substrates such as β-lipotropin (β-LPH), adrenocorticotropin (ACTH), proopiomelanocortin (POMC)3 and substance P4. Here we describe the complete amino acid sequence of rat submaxillary gland, tonin. Comparison of the sequence of 219 amino acids with other serine proteases, particularly kallikreins, γ-subunit of nerve growth factor (NGF) and the recently described γ-renin, reveals extensive similarities. More interestingly, it also reveals the substitution of an Asp residue always found in the serine protease active site triad (Asp, His, Ser) by a Leu residue. This unusual substitution does not seem to affect the proteolytic activity of the enzyme.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Demassieux, S., Boucher, R., Grisé, C. & Genest, J. Can. J. Biochem. 54, 788–795 (1976).
Thibault, G. & Genest, J. Biochim. biophys. Acta 660, 23–29 (1981).
Seidah, N. G. et al. Biochem. biophys. Res. Commun. 86, 1002–1013 (1979).
Chrétien, M. et al. FEBS Lett. 113, 173–176 (1980).
Seidah, N. G. et al. Can. J. Biochem. 56, 920–925 (1978).
Lazure, C. et al. Nature 292, 383–384 (1981).
Ikeda, M., Gutkowska, J., Thibault, G., Boucher, R. & Genest, J. Hypertension 3, 81–86 (1981).
Lazure, C., Dennis, M., Rochemont, J., Seidah, N. G. & Chrétien, M. Analyt. Biochem. 125, 406–414 (1982).
Tschesche, H. et al. in Kinins-II (eds Fujii, S., Moriya, H. & Suzuki, T.) 245–260 (Plenum, New York, 1979).
Richards, R. I., Catanzaro, D. F., Mason, A. J., Morris, B. J., Baxter, J. D. & Shine, J. J. biol. Chem. 257, 2758–2761 (1982).
Lazure, C., Seidah, N. G., Thibault, G., Genest, J. & Chrétien, J. in Proceedings of the seventh American Peptide Symposium (eds Rich, D. H. & Gross, E.) 517–519 (Pierce Chemical, New York, 1981).
Swift, G. H., Dagorn, J. C., Ashley, P. L., Cummings, S. W. & MacDonald, R. J. Proc. natn. Acad. Sci. U.S.A. 79, 7263–7268 (1982).
Thomas, K. A., Baglan, N. C. & Bradshaw, R. A. J. biol Chem. 256, 9156–9166 (1981).
Poe, M. et al. J. biol Chem. 258, 2209–2216 (1982).
Bothwell, M. A., Wilson, W. H. & Shooter, E. M. J. biol. Chem. 254, 7287–7294 (1979).
Thomas, K. A., Silverman, R. E., Jeng, I., Baglan, N. G. & Bradshaw, R. A. J. biol Chem. 256, 9147–9155 (1981).
Schenkein, I., Franklin, E. C. & Frangione, A. Archs Biochem. Biophys. 209, 57–62 (1981).
Genest, M. & Ptak, M. Int. J. Peptide Protein Res. 19, 420–431 (1982).
Polgar, L. & Bender, M. L. Proc. natn. Acad. Sci. U.S.A. 64, 1335–1342 (1969).
Kurosky, A. et al. Proc. natn. Acad. Sci. U.S.A. 77, 3388 (1980).
Dayhoff, M. D. (Ed.) in Atlas of Protein Sequence and Structure Vol. 5, Suppl. 3 (1978).
Jany, K. D., Bekelar, K., Pfleiderer, G. & Ishay, J. Biochem. biophys. Res. Commun. 110, 1–7 (1983).
Christie, D. L. & Gagnon, J. Biochem. J. 209, 61–70 (1983).
Eur. J. Biochem. 5, 151–153 (1968).
Bause, E. & Legler, G. Biochem. J. 195, 639–644 (1981).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lazure, C., Leduc, R., Seidah, N. et al. Amino acid sequence of rat submaxillary tonin reveals similarities to serine proteases. Nature 307, 555–558 (1984). https://doi.org/10.1038/307555a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/307555a0
This article is cited by
-
Molecular anatomy: Phyletic relationships derived from three-dimensional structures of proteins
Journal of Molecular Evolution (1990)
-
The cellular physiology of glandular kallikrein
Kidney International (1986)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.