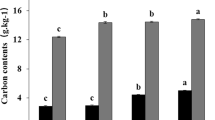
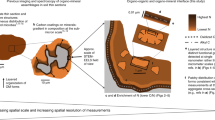
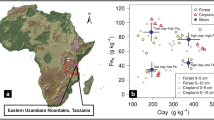
Abstract
The stability of humin, the alkali-resistant part of humic matter in soil1–4, is usually accounted for by its intimate association with inorganic soil colloids, especially swelling clays and iron compounds. Although the stability of these organo-clay complexes has been explained by interlamellar intercalation, there is evidence2–4 that large-sized humic acid nuclei are unlikely to penetrate layer silicates in normal soil conditions. The ability of clay minerals to promote polymerization of organic monomers has long been recognized5, and has been confirmed recently6–8; however, the conditions in which these catalytic properties were exhibited were unlike those existing in natural soils. We now report the results of preliminary investigations suggesting that humin-like substances may form between layers of silicate clays from smaller aromatic molecules in conditions that resemble those occurring naturally.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Schnitzer, M. & Khan, S. V. Humic Substances in the Environment (Marcel Decker, New York, 1972).
Duchaufour, P. Pédologie Vol. 1 (Masson, Paris, 1977).
Hayes, M. H. B. & Swift, R. S. in The Chemistry of Soil Constituents Ch. 3 (eds Greenland, D.J. & Hayes, M.H.B.) (Wiley, New York, 1978).
Theng, B. K. G. Formation and Properties of Clay-Polymer Complexes (Elsevier, Amsterdam, 1979).
Theng, B. K. G. The Chemistry of Clay Organic Reactions (Hilger, London, 1974).
Mortland, M. M. & Halloran, L. J. Am. J. Soil. Sci. Soc. 40, 367 (1976).
Stoessel, F., Guth, J. L. & Wey, R. Clay Miner. 12, 255 (1977).
Cloos, P., Moreale, A., Broers, C. & Badot, C. Clay Miner. 14, 307 (1979).
Moreale, A. & Van Bladel, R. Clay Miner 14, 1 (1979).
Mortland, M. M. & Pinnavaia, T. J. Nature phys. Sci. 229, 75 (1971).
Cloos, P., Vande Poel, D. & Camerlynck, J. P. Nature phys. Fi. 243, 54 (1973).
Pinnavaia, T. J., Hall, P. A., Cady, S. S. & Mortland, M. M. J. phys. Chem. 78, 994 (1974).
Rupert, P. J. J. phys. Chem. 77, 784 (1973).
Doner, H. E. & Mortland, M. M. Science 166, 1406 (1969).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Cloos, P., Badot, C. & Herbillon, A. Interlayer formation of humin in smectites. Nature 289, 391–393 (1981). https://doi.org/10.1038/289391a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/289391a0
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.