Abstract
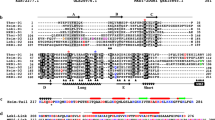
THE realisation that actin is involved not only in contractility in muscle tissue but also in general cytoplasmic movement and cell locomotion was based on the isolation of actin from lower eukaryotic organisms, such as Physarum polycephalum1, which show active protoplasmic streaming. It is now accepted that actin is the major structural protein of microfilaments characteristically found in the cytoplasm of most if not all higher cells, and that actins have been highly conserved during eukaryotic evolution and differentiation due to the constraints imposed by the large number of specific interactions which actin shows with other cytoplasmic proteins (for a review see ref. 2). Preliminary fingerprint analysis on Physarum actin3 and partial amino acid sequence analysis on actin from Acanthamoeba (ref. 4 and Elzinga cited in ref. 2) have emphasised this point, although so far a complete amino acid sequence has not been reported for actin from a lower eukaryotic species. Our studies on the primary structure of different mammalian actins have shown that several cytoplasmic actins are very similar if not identical in amino acid sequence, although they differ by at least 25 amino acids from the corresponding skeletal muscle actin5. The availability of fast amino acid sequence procedures for actin5 led us to study the actin from Physarum polycephalum to assess how cytoplasmic actins differ between lower and higher eukaryotes. We report here results which show that Physarum plasmodia, in contrast to non-muscle cells of higher vertebrates5–7, contain only one cytoplasmic actin species. This actin is more closely related to mammalian cytoplasmic actins than these actins are to skeletal muscle actin. The high degree of structural conservation of the actin sequence and structure during eukaryotic evolution is illustrated by the absence of any amino acid deletions or additions to the polypeptide chain beyond residue 2. Furthermore, the distribution of the proline residues remains unchanged and substitutions of amino acid residues with charged side chains are very rare, indicating a high conservation of the surface topography of the actin molecule.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Hatano, S. & Oosawa, F. Biochim. biophys. Acta 127, 488–498 (1966).
Korn, E. D. Proc. natn. Acad. Sci. U.S.A. 75, 588–599 (1978).
Jockusch, B. M., Becker, M., Hindennach, I. & Jockusch, H. Expl Cell Res. 89, 241–246 (1974).
Elzinga, M. & Lu, R. C. in Contractile Systems in Non-Muscle Tissues (eds Perry, S. V., Margreth, A. & Adelstein, R.) 29–37 (Elsevier, Amsterdam, 1976).
Vandekerckhove, J. & Weber, K. Eur. J. Biochem. 90, 451–462 (1978).
Whalen, R. G., Butler-Browne, G. S. & Gros, F. Proc. natn. Acad. Sci. U.S.A. 73, 2018–2022 (1976).
Garrels, J. I. & Gibson, W. Cell 9, 793–805 (1976).
Lazarides, E. & Lindberg, U. Proc. natn. Acad. Sci. U.S.A. 71, 4742–4746 (1974).
Zechel, K. & Weber, K. Eur. J. Biochem. 89, 105–112. (1978).
Vandekerckhove, J. & Weber, K. Proc. natn. Acad. Sci. U.S.A. 75, 1106–1110 (1978).
Weber, K., Koch, R., Herzog, W. & Vandekerckhove, J. Eur. J. Biochem. 78, 27–32 (1977).
Offord, R.E. Nature 211, 591–593 (1966).
Collins, J. H. & Elzinga, M. J. biol. Chem. 250, 5915–5920 (1975).
Lu, R. C. & Elzinga, M. Biochemistry 16, 5801–5806 (1977).
Weihing, R. R. & Korn, E. D. Biochemistry 10, 590–600 (1971).
Vandekerckhove, J. & Weber, K. J. molec. Biol. (in the press).
Eckert, B. S. & Lazarides, E. J. Cell Biol. 77, 714–721 (1978).
Gordon, D. J., Boyer, J. L. & Korn, E. D. J. biol. Chem. 252, 8300–8309 (1977).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
VANDEKERCKHOVE, J., WEBER, K. The amino acid sequence of Physarum actin. Nature 276, 720–721 (1978). https://doi.org/10.1038/276720a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/276720a0
This article is cited by
-
Developmental expression of the alpha-skeletal actin gene
BMC Evolutionary Biology (2008)
-
The yeast type II myosin heavy chain: Analysis of its predicted polypeptide sequence
Journal of Muscle Research and Cell Motility (1991)
-
The single-copy actin gene of Phytophthora megasperma encodes a protein considerably diverged from any other known actin
Plant Molecular Biology (1990)
-
Regulation of actin polymerization by non-polymerizable actin-like proteins
Nature (1984)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.