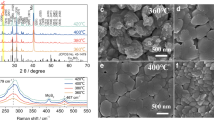
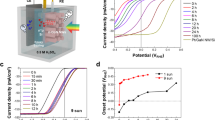
Abstract
FOLLOWING Fujishima and Honda's suggestion of water photolysis by a photoelectrochemical cell1, no stable cell has been reported (but compare refs 2, 3) capable of water decomposition without the application of either an external bias or a pH gradient. The main problems have been to find stable anodes and cathodes with sufficiently negative and positive critical photopotentials, respectively, so that they may be suitably combined in a self-driving photo cell producing hydrogen and oxygen from water. We report here that the anodes n-TiO2 and n-SrTiO3 and the cathodes p-CdTe and p-GaP can be combined to form four separate stable self-driven cells: n-TiO2–p-CdTe, n-TiO2–p-GaP, n-SrTiO3–p-CdTe and n-SrTiO3–p-GaP capable of water photoelectrolysis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Fujishima, A. & Honda, K. Nature 238, 37–38 (1972).
Mavroides, J. G., Tchernev, D. I., Kafalas, J. A. & Kolesar, D. F. Mater. Res. Bull. 10, 1023–1030 (1975).
Watanabe, T., Fujishima, A. & Honda, K. Bull. chem. Soc. Jap. 49, 355–358(1976).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
OHASHI, K., MCCANN, J. & BOCKRIS, J. Stable photoelectrochemical cells for the splitting of water. Nature 266, 610–611 (1977). https://doi.org/10.1038/266610a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/266610a0
This article is cited by
-
Photoelectrochemical technology for solar fuel generation, from single photoelectrodes to unassisted cells: a review
Environmental Chemistry Letters (2022)
-
A titanic breakthrough
Nature Catalysis (2021)
-
Electronic Structures of Free-Standing Nanowires made from Indirect Bandgap Semiconductor Gallium Phosphide
Scientific Reports (2016)
-
Semiconductor nanowires for photovoltaic and photoelectrochemical energy conversion
Frontiers of Physics (2014)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.