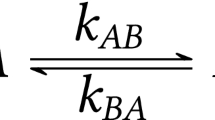Abstract
WE wish to report an application of the relationship between the kinetics of triplet energy transfer and donor–acceptor geometries to biomolecular systems. Our study illustrates the usefulness of measurements of this type in estimating molecular distances and providing a measure of variability within enzyme–inhibitor complexes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Emolaev, V. L., Soviet Phys. Usp., 80, 333–358 (1963).
Galley, W. C., Biopolymers, 6, 1279–1295 (1968).
Galley, W. C., and Stryer, L., Proc. natn. Acad. Sci. U.S.A., 60, 108–114 (1968).
Longworth, J. W., in Excited States of Proteins and Nucleic Acids (edit. by Steiner, R. F., and Weinryb, I.), 319–474 (Plenum, New York, 1971).
Ramachandran, N., and Chiron, C. A., Biophys. J., 13, 272 Abstr. (1973).
Chiron, C. A., Longworth, J. W., and Ramachandran, N., Proc. natn. Acad. Sci. U.S.A., 70, 3703–3706 (1973).
Matsuyama, A., and Nagata, C., Biochim. biophys. Acta, 224, 588–596 (1970).
Porter, G., and Wilkinson, F., Proc. R. Soc., Lond., A264, 1–18 (1961).
Anderson, R. W., Hochstrasser, R. M., Lutz, H., and Scott, G. W., J. chem. Phys., 61, 2500–2506 (1974).
Nieman, G. C., and Robinson, G. W., J. chem. Phys., 37, 2150–2151 (1972).
Kobashi, H., Morito, T., and Mataga, N., Chem. Phys. Lett., 20, 376–378 (1973).
Strambini, G. B., and Galley, W. C., J. chem. Phys., 63, 3467–3472 (1975).
Dexter, D. L., J. chem. Phys., 21, 836–850 (1953).
Strambini, G. B., thesis, McGill Univ. (1975).
Kannan, K. K., et al., Cold Spring Harb. Symp. quant. Biol., 36, 221 (1971).
Small, J. G., and Ashari, R., Rev. Sci. Instrum., 43, 1205–1206 (1972).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
GALLEY, W., STRAMBINI, G. Kinetics of triplet–triplet energy transfer and intramolecular distances in enzyme–inhibitor complexes. Nature 261, 521–522 (1976). https://doi.org/10.1038/261521a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/261521a0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.