Abstract
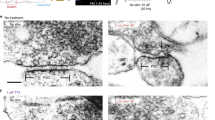
PINCHED-OFF presynaptic nerve terminals (synaptosomes) prepared from rat brain homogenates1,2 retain many of the functional properties of intact neurones3,4. They can accumulate K+ and extrude Na+ against concentration gradients5,6, and their membranes seem to develop K+ diffusion potentials which behave like the membrane potentials of most neurones7. Furthermore, the isolated terminals exhibit an increased rate of Ca2+ accumulation8 and Ca2+-dependent transmitter release8–10 when exposed to depolarising agents such as veratridine or an increased external K+ concentration ([K]0). The latter findings are consistent with the ‘calcium hypothesis’ of depolarisation–secretion coupling11. The calcium which enters the terminals during depolarisation seems to trigger the release of transmitter from intraterminal vesicles by an exocytotic process involving transient fusion of the vesicle membrane with the surface membrane12–14. This hypothesis is supported by experiments with electron-opaque extracellular markers; the appearance of the markers within intraterminal vesicles, after stimulation, has been taken as evidence for vesicle recycling15–17. Here we report similar observations on synaptosomes incubated in vitro, showing that synaptosomes function like intact terminals in this respect as well.
This is a preview of subscription content, access via your institution
Access options
Similar content being viewed by others
References
DeRobertis, E., Pellegrino deIraldi, A., Rodriguez deLores Arnaiz, G., and Salganicoff, L., J. Neurochem., 9, 23–35 (1962).
Gray, E. G., and Whittaker, V. P., J. Anat., 96, 79–88 (1962).
Rodriguez deLores Arnaiz, G., and DeRobertis, E., Curr. Top. Membr. Trans., 3, 237–272 (1972).
Bradford, H. F., Handbook of Psychopharmacology, 1 (edit. by Iverson, L. L. Iverson, S. D., and Synder, S. H.), 191–252 (Plenum, New York, 1975).
Escueta, A. V., and Appel, S. H., Biochemistry, 8, 725–733 (1969).
Ling, C.-M., and Abdel-Latiff, A. A., J. Neurochem., 15, 721–730 (1968).
Blaustein, M. P., and Goldring, J. M., J. Physiol., Lond., 247, 589–615 (1975).
Blaustein, M. P., J. Physiol., Lond., 247, 617–655 (1975).
Blaustein, M. P., Johnson, E. M., Jr., and Needleman, P., Proc. natn. Acad. Sci. U.S.A., 69, 2237–2240 (1972).
De Belleroche, J. S., and Bradford, H. F., J. Neurochem., 19, 1817–1819 (1972).
Katz, B., and Miledi, R., J. Physiol., Lond., 203, 459–487 (1969).
Douglas, W. W., Nagasawa, J., and Schultz, R. A., Nature, 227, 407–409 (1970).
Pfenninger, K. H., and Rovainen, C. M., Brain Res., 72, 1–23 (1974).
Heuser, J. E., Reese, T. S., and Landis, D. M. D., J. Neurocytol., 3, 109–131 (1974).
Ceccarelli, B., Hurlbut, W. P., and Mauro, A., J. Cell Biol., 57, 499–524 (1973).
Heuser, T. E., and Reese, T. S., J. Cell Biol., 57, 315–344 (1973).
Turner, P. T., and Harris, A. B., Nature, 242. 57–59 (1973).
Blaustein, M. P., and Ector, A. C., Biochim. biophys. Acta, 419, 295–308 (1976).
Graham, R., and Karnovsky, M., J. Histochem. Cytochem., 14, 291–302 (1965).
Karnovsky, M. J., Am. Soc. Cell Biol., 11th Mtg, Abstr., 146 (1971).
Gray, E. G., and Willis, R. A., Brain Res., 24, 149–168 (1970).
McMahan, U. J., and Yee, A., Neurosci. Abstr., Soc. Neurosci., Fifth Ann. Mtg., 624 (1975).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
FRIED, R., BLAUSTEIN, M. Synaptic vesicle recycling in synaptosomes in vitro. Nature 261, 255–256 (1976). https://doi.org/10.1038/261255a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/261255a0
This article is cited by
-
Time course and frequency dependence of synaptic vesicle depletion and recovery in electrically stimulated sympathetic ganglia
Journal of Neurocytology (1987)
-
Electron microscope investigation of synapses, synapse-like structures, and possible sites of release of neurosecretory material in the buccal ganglia of Helix pomatia (Mollusca)
Zoomorphology (1982)
-
An association between mitochondria and microtubules in synaptosomes and axon terminals of cerebral cortex
Journal of Neurocytology (1978)
-
Immunochemical comparison of synaptic plasma membrane and synaptic vesicle membrane antigens
Journal of Neurocytology (1977)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.