Abstract
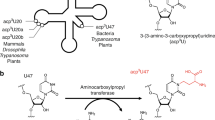
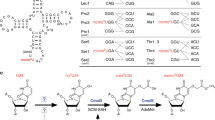
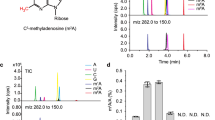
RIBOTHYMIDINE (m5U or rT), the most common methylated nucleoside found in tRNA, is always at the 23rd position from the 3′ end in the nucleotide sequence GTψCPurine1,2. So far ribothymidine has been found in essentially all pro-karyotic tRNAs which function in protein synthesis, but not in several eukaryotic ‘elongator’ tRNAs3–6. In the case of wheat germ, all the glycine tRNAs, several species of threonine tRNA and one species of tyrosine tRNA have an unmodified uridine in the position usually occupied by ribothymidine (ref. 5 and K.B.M., D. Marcu, and B.S.D., manuscript in preparation). To determine the significance of the absence of rT in these tRNAs, we have methylated them enzymatically with a crude preparation of E. coli rT-forming uracil methyltransferase and then compared their efficiency with that of the unmodified tRNAs in a wheat germ cell-free protein synthesising system directed by various natural mRNAs. We report here that these tRNAs function significantly more efficiently in protein synthesis when lacking rT and that the inhibitory effect of rT can be reversed by the polyamine spermine.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Kim, S. H., et al., Proc. natn. Acad. Sci. U.S.A., 71, 4970–4974 (1974).
Nishimura, S., in Biochemistry of Nucleic Acids (edit. by Burton, K.), 289–322 (Butterworths, London, 1974).
Kimura-Haroda, F., Saneyoshi, M., and Nishimura, S. FEBS Lett., 13, 335–338 (1971).
Faras, A. J., et al., J. Virol., 13(5), 1134–1142 (1974).
Marcu, K., et al., Biochem. biophys. Res. Commun., 55, 477–483 (1973).
Piper, R. W., and Clark, B. F. C., FEBS Lett., 47, 56–59 (1974).
Ghosh, K., Ghosh, H. P., Simsek, M., and RajBhandary, U. L., J. Biol. Chem., 249, 4720–4729 (1974).
Simsek, M., RajBhandary, U. L., Boisnard, M., and Petrissant, G., Nature, 247, 518–520 (1974).
Simsek, M., Petrissant, G., and RajBhandary, U. L., Proc. natn. Acad. Sci U.S.A., 70, 2600–2604 (1973).
Piper, P. W., and Clark, B. F. C., FEBS Lett., 30, 265–267 (1973).
Simsek, M., and RajBhandary, U. L., Biochem. biophys. Res. Commun., 49, 508–515 (1972).
Roberts, R. J., Nature new Biol., 237, 44–45 (1972).
Delk, A. S., and Rabinowitz, J. C., Nature, 252, 106–109 (1974).
Ofengand, J., and Henes, C., J. biol. Chem., 244, 6241–6253 (1969).
Schwarz, U., Luhmann, R., and Gassen, H. B., Biochem. biophys. Res. Commun., 56, 807–814 (1974).
Erdmann, V. A., Sprinzl, M., and Pongs, O., Biochem. biophys. Res. Commun., 54, 942–948 (1973).
Littlefield, J. W., and Dunn, D. B., Biochem. J., 70, 642–651 (1958).
Marcu, K., and Dudock, B., Nucleic Acid Res., 1, 1385–1397 (1974).
Roberts, B. E., Mathews, M. B., and Burton, C. J., J. molec. Biol., 80, 733–742 (1973).
Efron, D., and Marcus, A., Virology, 53, 343–348 (1973).
Shih, D., and Kaesberg, P., Proc. natn. Acad. Sci. U.S.A., 70, 1799–1803 (1973).
Paul, M., Goldsmith, M. R., Hunsley, J. R., and Kafatos, F. C., J. Cell Biol., 55, 653–680 (1972).
Marcus, A., in Protein Biosynthesis in Nonbacterial Systems (edit. by Last, J. A. and Laskin, A. L.), 127–145 (Dekker, New York, 1972).
Allende, J. E., in Techniques in Protein Biosynthesis, 2 (edit. by Campbell, P. N., and Sargent, J. R.), 55–100 (Academic, New York, 1969).
Allen, E. H., and Schweet, R. S., J. biol. Chem., 237, 760–767 (1962).
Kemper, B., Habener, J. F., Mulligan, R. C., Potts, J. T., Jr, and Rich, A., Proc. natn. Acad. Sci. U.S.A., 71, 3731–3735 (1974).
Konecki, D., Gisela, K., Pinphanichakarn, P., and Hardesty, B., Archs Biochem. Biophys., 169, 192–198 (1975).
Atkins, J. F., Lewis, J. B., Anderson, C. W., and Gesteland, R. F., J. biol. Chem, 250, 5688–5695 (1975).
Johnson, H. G., and Bach, M. K., Archs Biochem. Biophys., 128, 113–123 (1968).
Cohen, S. S., Ann. N. Y. Acad. Sci., 171, 869–881 (1970).
Cohen, S. S., Introduction to the Polyarnines (Prentice Hall, Englewood Cliffs, New Jersey, 1971).
Cohen, S. S., Morgan, S., and Streibel, E., Proc. natn. Acad. Sci. U.S.A. 64, 669–676 (1969).
Schrier, A., and Schimmel, P., J. molec. Biol., 93, 323–330 (1975).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
MARCU, K., DUDOCK, B. Effect of ribothymidine in specific eukaryotic tRNAs on their efficiency in in vitro protein synthesis. Nature 261, 159–162 (1976). https://doi.org/10.1038/261159a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/261159a0
This article is cited by
-
Analysis of the complicated dynamics of some harvesting models
Journal of Mathematical Biology (1987)
-
Codon binding and translational properties of an isoaccepting lysine tRNA peculiar to virus-transformed cells
Molecular and General Genetics MGG (1981)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.