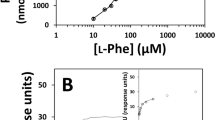
Abstract
THE molecular defect in classical phenylketonuria (PKU) has not been elucidated, but it seems certain that the block in metabolism is in the conversion of phenylalanine to tyrosine1 and that the enzyme which carries out this reaction, phenylalanine hydroxylase (EC, 1.14.3.1), is defective2. Progress in delineating the molecular defect has been slow because of the limited tissue distribution of phenylalanine hydroxylase, lability of this enzyme after death3, scarcity of normal and PKU livers for study and the nonspecific purification methods for this enzyme. We recently developed a specific, one-step method for purification of phenylalanine hydroxylase from crude extracts of monkey liver4, and have now applied it to crude extracts of livers from two human foetuses, from three patients who died of unrelated disease and from a patient with classical PKU who died at 12 yr. (The PKU patient did not follow the rapidly progressive course described recently in variant forms of PKU5 and dihydropteridine reductase activity was demonstrated in the liver.)
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Jervis, G. A., J. biol. Chem., 169, 651–656 (1947).
Grimm, U., Knapp, A., Schlenzka, K., and Reddeman, H., Clin. chim. Acta, 58, 17–21 (1975).
Mitoma, C., Auld, R. M., and Udenfriend, S., Proc. Soc. exp. Biol. Med., 94, 634–635 (1957).
Cotton, R. G. H., and Grattan, P., Eur. J. Biochem., 60, 427–430 (1975).
Butler, I. J., et al., Pediat. Res., 9, 348 (1975).
Margolis, J., and Kenrick, K. G., Nature, 214, 1334–1336 (1967).
Kaufman, S., and Fisher, D. B., in Molecular Mechanisms of Oxygen Activation, (edit. by Hayaishi, O.), 285–369 (Academic, New York, 1974).
Connellan, J. M., and Danks, D. M., Biochim. biophys. Acta, 293, 48–55 (1973).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
COTTON, R., DANKS, D. Purification of inactive phenylalanine hydroxylase protein from liver in classical phenylketonuria. Nature 260, 63–64 (1976). https://doi.org/10.1038/260063a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/260063a0
This article is cited by
-
Phenylketonuria
The Science of Nature (1980)
-
Genetics of the mammalian phenylalanine hydroxylase system. IV. Evidence of phenylalanine hydroxylase in a cultured human hepatoma cell line
Biochemical Genetics (1980)
-
Observations indicating the nature of the mutation in phenylketonuria
Journal of Inherited Metabolic Disease (1979)
-
Genetics of the mammalian phenylalanine hydroxylase system. II. Immunological and two-dimensional gel electrophoretic studies of phenylalanine hydroxylase in cultured normal and mutant rat hepatoma cells
Biochemical Genetics (1979)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.