Abstract
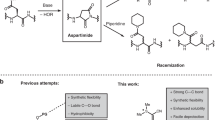
PORCINE insulin has only three amino groups, those of the terminal residues of the two chains (glycineA1, phenylalanineB1) and a side chain amino group (lysineB29)1. Many reagents have been developed to take advantage of the reactivity of the NH2 group in proteins—probably more than for any other functional group—and there have been many reports on the modification of insulin activity by such reagents. Our approach is part of an attempt to develop and use methods which make it unnecessary to synthesize a whole protein when a structure is required which departs only slightly from a naturally occurring one2. In the present application to insulin it resembles that of Brandenburg and Ooms3, who used Edman's phenylisothiocyanate (PITC) method4 to attack and remove tho amino terminal residues of both chains and subsequently coupled an amino-acid back in their place. In this way residues A1 and B1 had both to be replaced with the same amino-acid (glycine). In the course of the procedure, lysineB29 was (irreversibly) protected by acetylation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Brown, H., Sanger, F., and Kitai, R., Biochem. J., 60, 556 (1955).
Offord, R. E., Nature, 221, 37 (1969).
Brandenburg, D., and Ooms, H. A., Proc. Intern. Symp. Protein and Polypeptide Hormones, Liége, 482 (1968).
Edman, P., Acta Chem. Scand., 4, 277 (1950).
Wrigley, C., Science Tools, 15, 17 (1968).
Brandenburg, D., Z. Physiol. Chem., 350, 741 (1969).
Nefkens, G. H. L., Tesser, G. I., and Nivaud, R. J. F., Rec. Trav. Chim. Pays-Bas Belg., 79, 688 (1960).
Bodanszky, M., and Ondetti, M., J. Amer. Chem. Soc., 86, 4452 (1964).
Schwyzer, R., Costopanagiotis, A., and Sieber, P., Chimia, 16, 295 (1962).
Schallenberg, E. E., and Calvin, A., J. Amer. Chem. Soc., 77, 2779 (1955).
Goldberger, E., and Anfinsen, C. B., Biochemistry, 1, 401 (1964).
Weygand, F., and Röpsch, A., Chem. Ber., 92, 2095 (1959).
Foens-Bech, P., and Damkjaer, M., Rep. Steno. Mem. Hosp. Nord. Insulin. Lab., 10, 127 (1961).
Schlichtkrull, J., Acta Chem. Scand., 10, 1455 (1956).
Schlichtkrull, J., thesis (Univ. of Copenhagen, 1958).
Du, Y-C., Zhang, Y-S., Lu, Z., and Tsou, C-L., Scientia Sinica, X, 84 (1961).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
BORRÁS, F., OFFORD, R. Protected Intermediate for the Preparation of Semisynthetic Insulins. Nature 227, 716–718 (1970). https://doi.org/10.1038/227716a0
Received:
Issue Date:
DOI: https://doi.org/10.1038/227716a0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.