Abstract
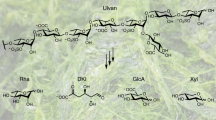
CARRAGEENANS function in the intercellular regions of certain red seaweeds1–3 and have residues of two types, designated A (α-linked) and B (β-linked), which are arranged alternately and may themselves occur as different variants. When A is 3,6-anhydro-D-galactose 2-sulphate and B is D-galactose 4-sulphate, the structure is an idealization known as ι-carrageenan4. Another member of the family, ϰ-carrageenan, has the same structure except that the 2-positions carry little or no sulphate. Native polysaccharides usually depart from these idealizations in having a proportion of A residues in the form of 6-sulphate (I)5,6. Evidence from X-ray diffraction7 and optical rotation4,8,9 indicates that the gel framework is formed by cross-linking of chains in double helices, and that the 6-sulphate residues must represent8 impediments to this process, or “kinks” in the helix strand; schematically, the chain is IV rather than III. It is tempting to propose that these kinks are used to regulate the extent of helix formation, and therefore the physical and biological properties7. We report here a search for an enzyme which would be involved in such control.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Rees, D. A., Adv. Carbohydrate Chem., 24, 267 (1969).
Rees, D. A., Brit. Phycol. Bull., 2, 180 (1962).
Eppley, R. W., and Cyrus, C. C., Biol. Bull., Woods Hole, 118, 55 (1960).
Rees, D. A., Scott, W. E., and Williamson, F. B., Nature, 227, 390 (1970) (preceding article).
Anderson, N. S., Dolan, T. C. S., and Rees, D. A., J. Chem. Soc., C, 596 (1968).
Anderson, N. S., Dolan, T. C. S., Penman, A., Rees, D. A., Mueller, G. P., Stancioff, D. J., and Stanley, N. F., J. Chem. Soc., C, 602 (1968).
Anderson, N. S., Campbell, J. W., Harding, M. M., Rees, D. A., and Samuel, J. W. B., J. Mol. Biol., 45, 85 (1969).
Rees, D. A., Steele, I. W., and Williamson, F. B., J. Polymer Sci., C, 28, 261 (1969).
McKinnon, A. A., Rees, D. A., and Williamson, F. B., Chem. Commun., 701 (1969).
Anderson, N. S., Dolan, T. C. S., Lawson, C. J., Penman, A., and Rees, D. A., Carbohydrate Res., 7, 468 (1968).
Yaphe, W., Anal. Chem., 32, 1327 (1960).
Rees, D. A., Biochem. J., 81, 347 (1961).
Rees, D. A., and Conway, E., Biochem. J., 84, 411 (1962).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
LAWSON, C., REES, D. An Enzyme for the Metabolic Control of Polysaccharide Conformation and Function. Nature 227, 392–393 (1970). https://doi.org/10.1038/227392a0
Received:
Issue Date:
DOI: https://doi.org/10.1038/227392a0
This article is cited by
-
Advances in single-molecule junctions as tools for chemical and biochemical analysis
Nature Chemistry (2023)
-
Concise review of industrially important red seaweed Gracilaria dura (C. Agardh) J. Agardh
Journal of Applied Phycology (2022)
-
Effects of additives on the lyophilized and thermal stability of d-galactose-6-sulfurylase activity from Eucheuma striatum (Rhodophyta)
Journal of Applied Phycology (2015)
-
How do giant plant cells cope with injury?—The wound response in siphonous green algae
Protoplasma (1988)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.