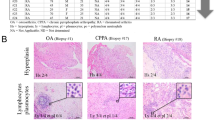
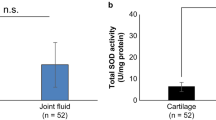
Abstract
ONE subcellular manifestation of rheumatoid arthritis is that the membranes of the lysosomes in the synovial lining cells are virtually functionless; by contrast, in non-rheumatoid synovia—even after recent mechanical trauma—the lysosomal membranes in the lining cells are functional in that they retard entry of the substrates used for the demonstration of the intra-lysosomal enzymes1. Barrett and Poole2, while not disputing this conclusion, questioned the precise nature of the lysosomal enzyme used in the study, which was visualized and measured microdensitometrically on the basis of the hydrolysis of leucine-2-naphthylamide, the liberated naphthylamine being trapped by ‘Fast Blue B’ (tetra-azotized dianisidine) to form an insoluble dye. By precise biochemical analysis, they demonstrated that human synovial tissue, taken from rheumatoid joints, contained cathepsins B and D as well as aminopeptidase activity. They showed that cathepsin D cannot hydrolyse leucine-2-naphthylamide and quoted unpublished data from other workers which suggested that cathepsin B could not hydrolyse this substrate. From this evidence they concluded that the particulate enzyme activity which was demonstrated by this substrate was a lysosomal aminopeptidase.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Chayen, J., Bitensky, L., Butcher, R. G., and Poulter, L. W., Nature, 222, 281 (1969).
Barrett, A. J., and Poole, A. R., Nature, 224, 279 (1969).
Snellman, O., Biochem. J., 114, 673 (1969).
Barland, P., Novikoff, A. B., and Hamerman, D., Amer. J. Pathol., 44, 853 (1964).
Ghadially, F. N., and Roy, S., Ann. Rheum. Dis., 26, 426 (1967).
Wyllie, J. C., Haust, M. D., and More, R. H., Lab. Invest., 15, 519 (1966).
Chayen, J., Bitensky, L., Butcher, R. G., and Poulter, L. W., A Guide to Practical Histochemistry (Oliver and Boyd, Edinburgh, 1969).
Butcher, R. G., Poulter, L. W., and Chayen, J., Biochem. J., 107, 27P (1968).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
CHAYEN, J., BITENSKY, L. & POULTER, L. Histochemistry of some Lysosomal “Proteolytic” Enzymes of Human Rheumatoid Synovia. Nature 225, 1050–1051 (1970). https://doi.org/10.1038/2251050a0
Received:
Issue Date:
DOI: https://doi.org/10.1038/2251050a0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.