Abstract
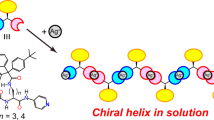
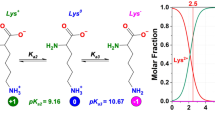
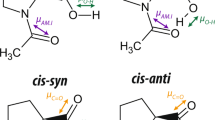
THE need to use non-aqueous solvents has limited the biological value of experimental work on many synthetic polypeptides. Considerable interest has therefore been shown in their surface chemistry, because, quite apart from the importance of understanding the conformation of polypeptides at interfaces, the properties of the polymer when exposed to water can also be investigated. From a combination of the classical methods of surface chemistry with the normal methods of structural investigation, there is at present good evidence to show that the α-helix is present in molecular monolayers of a number of synthetic polypeptides at the air/water interface1. This work has been extended to poly-β-benzyl-L-aspartate, and in this communication I consider chiefly the properties of the polymer after removal from the water surface; full details of the surface chemistry will be given elsewhere. In contrast to most synthetic polypeptides composed of L-residues, this polymer normally exists in solution and in the solid state as a left-handed rather than a right-handed α-helix2,3. If a specimen is heated to 160° C it undergoes a transition to the ω-helix, containing four residues per turn, which also appears to be left-handed4. Orientated specimens have characteristic infrared absorption frequencies and dichroism enabling the two conformations to be clearly recognized and distinguished from the right-handed α-helix4.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Malcolm, B. R., Proc. Roy. Soc., A, 305, 363 (1968).
Bradbury, E. M., Downie, A. R., Elliott, A., and Hanby, W. E., Proc. Roy. Soc., A, 259, 110 (1960).
Karlson, R. H., Norland, K. S., Fasman, G. D., and Blout, E. R., J. Amer. Chem. Soc., 82, 2268 (1960).
Bradbury, E. M., Brown, L., Downie, A. R., Elliott, A., Fraser, R. D. B., and Hanby, W. E., J. Mol. Biol., 5, 230 (1962).
Bradbury, E. M., Carpenter, B. G., and Stephens, R. M., Biopolymers, 6, 905 (1968).
Goodman, M., Felix, A. M., Deber, C. M., Brause, A. R., and Schwartz, G., Biopolymers, 1, 371 (1963).
Ooi, T., Scott, R. A., Vanderkooi, G., and Sheraga, H. A., J. Chem. Phys., 46, 4410 (1967).
Blout, E. R., and Lenormant, H., Nature, 179, 960 (1957).
Elliott, A., Malcolm, B. R., and Hanby, W. E., Nature, 179, 960 (1957).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
MALCOLM, B. Right-handed α-Helix and Conformational Changes in Poly-β-benzyl-L-aspartate. Nature 219, 929–930 (1968). https://doi.org/10.1038/219929a0
Received:
Issue Date:
DOI: https://doi.org/10.1038/219929a0
This article is cited by
-
Orientation of Polypeptide Side Chains in Solution
Nature (1970)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.