Abstract
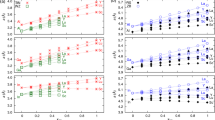
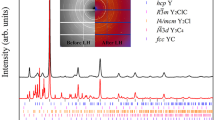
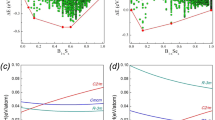
THE occurrence of such PbFCl-type phases as ThAs2, ThSb2 and UP2 led me to suspect that analogous rare earth compounds LnYX (X = chalcogen, Y = pnigogen) might also exist. I tried to synthesize a number of these hypothetical compounds either by reacting the elements or by sintering pressed mixtures LnY + X at temperatures of between 600° and 1,000° C. PbFCl-type phases were obtained for LnSbTe (Ln = La … Sm, Gd … Tm, Y). The samples, however, appeared to be antimony-deficient. Sb- and As-deficient samples of LaAsSe, LnSbS (Ln = La … Nd) and LaSbSe also showed the X-ray pattern characteristic of the PbFCl structure. The compounds LnYX with the lighter anions, however, revealed slightly different X-ray patterns. The symmetry of the tetragonal PbFCl cell is lowered to orthorhombic because of a small difference between the a and b axes. The lattice constants of GdAsSe are: a = 3.99 Å, b = 3.95 Å, c = 8.77 Å. Knowledge of the the electrical conductivity might give information regarding the distortion of the lattice; thus non-metallic properties are possible only if two electrons per formula unit are involved in anion–anion bonds and these bonds would give rise to specific distortions. Undistorted PbFCl-type LnYX compounds must be metallic. Unfortunately, the quality of the samples was not high enough to decide unequivocally whether these phases, if stoichiometric, are semiconductors or metals. Seebeck coefficients are of the order of 10–30 μV/°C and the resistivities are rather low. GdAsSe is antiferromagnetic with a Néel temperature of 10° K.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Wang, R., and Steinfink, H., Inorg. Chem., 6, 1685 (1967).
Wang, R., Bodnar, R. E., and Steinfink, H., Inorg. Chem., 5, 1468 (1966).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
HULLIGER, F. GdPS and Related New Rare Earth Compounds. Nature 219, 373–374 (1968). https://doi.org/10.1038/219373a0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1038/219373a0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.