Abstract
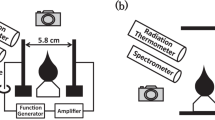
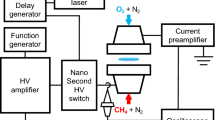
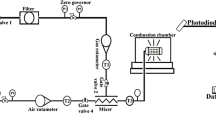
WE wish to comment on an omission of generality in the interpretation of data obtained by Green and Sugden1 concerning ionization in flames which contain hydrocarbons. These data form the quantitative basis for the reaction  which is usually accepted to be responsible for CHO+ in hydrocarbon flames2–7. With premixed flames of hydrogen, oxygen and nitrogen at atmospheric pressure, and a < 1 per cent admixture of C2H2, Green and Sugden analysed their data according to the general mechanism
which is usually accepted to be responsible for CHO+ in hydrocarbon flames2–7. With premixed flames of hydrogen, oxygen and nitrogen at atmospheric pressure, and a < 1 per cent admixture of C2H2, Green and Sugden analysed their data according to the general mechanism  This mechanism requires that [AH+] ∝ [H3O+]2max; the only ion observed which fits this criterion for a primary ion was found to be CHO+. Furthermore, the rate of ion formation was found to be proportional to [C2H2]0, thus B was assumed to be the only species containing carbon, C being a normal constituent of hydrogen–oxygen flames such as H, OH or O. Of the three possibilities for the formation of CHO+, C + OH, CO + H, and CH + O, the last was chosen as the most favourable.
This mechanism requires that [AH+] ∝ [H3O+]2max; the only ion observed which fits this criterion for a primary ion was found to be CHO+. Furthermore, the rate of ion formation was found to be proportional to [C2H2]0, thus B was assumed to be the only species containing carbon, C being a normal constituent of hydrogen–oxygen flames such as H, OH or O. Of the three possibilities for the formation of CHO+, C + OH, CO + H, and CH + O, the last was chosen as the most favourable.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Green, J. A., and Sugden, T. M., Ninth Symp. on Combustion, 607 (Academic Press, New York, 1963).
De Jaegere, S., Deckers, J., and Van Tiggelen, A., Eighth Symp. on Combustion, 155 (Williams and Wilkins, Baltimore, 1963).
Calcote, H. F., Eighth Symp. on Combustion, 184 (Williams and Wilkins, Baltimore, 1963).
Calcote, H. F., Kurzius, S. C., and Miller, W. J., Tenth Symp. on Combustion, 605 (The Combustion Institute, Pittsburgh, 1965).
Fontijn, A., Miller, W. J., and Hogan, J. M., Tenth Symp. on Combustion 545 (The Combustion Institute, Pittsburgh, 1965).
Hand, C. W., and Kistiakowsky, G. B., J. Chem. Phys., 37, 1239 (1962).
Bulewicz, E. M., and Padley, P. J., Ninth Symp. on Combustion, 638 (Academic Press, New York, 1963).
Glass, G. P., Kistiakowsky, G. B., and Michael, J. V., J. Chem. Phys., 42, 608 (1965).
Hand, Clifford W., and Kistiakowsky, G. B., J. Chem. Phys., 37, 1239 (1962).
Sternberg, J. C., Gallaway, W. S., and Jones, D. T. L., Gas Chromatography, 231 (Academic Press, New York, 1962).
Sugden, T. M., Annual Rev. Phys. Chem., 13, 369 (1962).
Vaidya, W. M., Proc. Roy. Soc., A, 147, 513 (1934).
Spokes, G. N., and Gaydon, A. G., Nature, 180, 1114 (1958).
Cummings, G. A. McD., and Hutton, E., Combustion and Flame, 10, 195 (1966).
Bulewicz, E. M., and Padley, P. J., Combustion and Flame, 5, 331 (1961).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
KLEMM, R., BLADES, A. Ionization in Hydrocarbon Flames. Nature 212, 920–921 (1966). https://doi.org/10.1038/212920a0
Issue Date:
DOI: https://doi.org/10.1038/212920a0
This article is cited by
-
Thermal and chemical ionization in flames
Combustion, Explosion, and Shock Waves (1970)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.