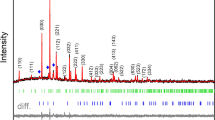
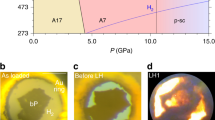
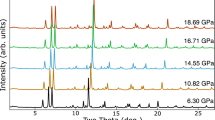
Abstract
PREVIOUS investigations1–5 of the niobium–phosphorus and tantalum–phosphorus systems indicate that only two intermediate phases occur in each system, a monophosphide (which shows a varying amount of stacking disorder in the structure) and a diphosphide. These results are based on investigations of the products formed when the metals react with phosphorus at temperatures below 1,100° C. In the tungsten–phosphorus system, the occurrence of tungsten monophosphide and a high and a low temperature form of tungsten diphosphide has been observed6–9. No further intermediate phases appear to be formed by direct reaction between tungsten and phosphorus below 1,100° C. It has been reported, however, that tungsten phosphides containing less than 50 atom per cent phosphorus can be prepared by fused salt electrolysis. Thus, Hsu et al.10 obtained a crystalline compound of composition W3P by electrolysis of a fused sodium metaphosphate bath containing tungsten trioxide and sodium chloride at temperatures below 900° C. This compound was described as having the body-centred tetragonal iron triphosphide-type structure with cell dimensions a = 9.890 Å; c = 4.808 Å.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Zumbusch, M., and Biltz, W., Z. Anorg. Chem., 246, 35 (1941).
Reinecke, A., Wiechmann, F., Zumbusch, M., and Biltz, W., Z. Anorg. Chem., 249, 14 (1942).
Zumbusch, M., and Biltz, W., Z. Anorg. Chem., 249, 20 (1942).
Schönberg, N., Acta Chem. Scand., 8, 226 (1954).
Boller, H., and Parthé, E., Acta Cryst., 16, 1095 (1963).
Faller, F. E., Biltz, W., Meisel, K., and Zumbusch, M., Z. Anorg. Chem., 248, 209 (1941).
Rundqvist, S., and Lundström, T., Acta Chem. Scand., 17, 37 (1963).
Gingerich, K. A., J. Phys. Chem., 68, 768 (1964).
Hulliger, F., Nature, 204, 775 (1964).
Hsu, S. S., Yocom, P. N., and Cheng, T. C. C., Interim Rep. Office of Naval Research Contract N 6-ori-71(50) (University of Illinois, Urbana, 1955).
Sellberg, B., and Rundqvist, S., Acta Chem. Scand., 19, (1965).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
RUNDQVIST, S. New Metal-rich Phosphides of Niobium, Tantalum and Tungsten. Nature 211, 847–848 (1966). https://doi.org/10.1038/211847a0
Issue Date:
DOI: https://doi.org/10.1038/211847a0
This article is cited by
-
Prediction of high-pressure phases of Weyl semimetal NbAs and NbP
Scientific Reports (2017)
-
Hydrothermal synthesis of cobalt–nickel bimetallic phosphides
Applied Nanoscience (2012)
-
Hydrothermal synthesis method of nickel phosphide nanoparticles
Applied Nanoscience (2012)
-
Investigation of the ternary systems W-B-P and W-Si-P in the range 0?0.66 P
Soviet Powder Metallurgy and Metal Ceramics (1984)
-
�ber Polyphosphide von Chrom, Molybd�n und Wolfram
Monatshefte f�r Chemie Chemical Monthly (1983)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.