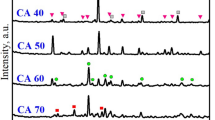
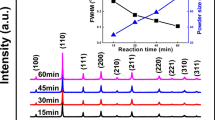
Abstract
SEVERAL methods for the preparation of the carbides of iron have been reported in the literature. In order to obtain a satisfactory product it may be necessary to use a relatively high temperature1, or long periods of reaction during which reaction conditions must be controlled within narrow limits for several weeks2,3. Using these methods we found considerable difficulty in preparing a sample of iron carbide which was sufficiently pure to be used in a projected study. During preliminary investigations of reactions of transition metal salts of organic acids, however, it was noticed that the solid residue from the thermal decomposition in vacuum, at about 500° C, of a ferric salt of mellitic acid gave an X-ray diffraction pattern characteristic of cementite. This communication is concerned with the use of this compound for the preparation of cementite. A detailed report of the kinetics of the thermal decomposition reaction will be published elsewhere4. Studies of the thermal decomposition of ferric salts of benzoic and of phthalic acids showed that no detectable cementite was present in the non-volatile crystalline products5.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Ephraim, F., Inorganic Chemistry, 869 (Gurney and Jackson, 1949).
Bahr, H. A., and Jessen, V., Ber., 66, 1238 (1933).
Hofer, L. J. E., The Preparation and Properties of Metal Carbides (U.S. Bureau of Mines R.I. 3770, 1944).
Galwey, A. K. (to be published).
Galwey, A. K., J. Chem. Soc., 4235 (1965).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
FREEL, J., GALWEY, A. A Preparation of Cementite. Nature 208, 183–184 (1965). https://doi.org/10.1038/208183a0
Issue Date:
DOI: https://doi.org/10.1038/208183a0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.