Abstract
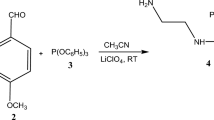
IT has previously been shown1 that in the presence of barium producing insoluble barium manganate, the decomposition of permanganate is of the first order with respect to: (a) the permanganate and (b) the hydroxide ion concentration. In the absence of barium the initial rate of decomposition as determined by means of the rotating disk method was found to be proportional to (a) the permanganate concentration, (b) the square of the hydroxide concentration, and (c) the reciprocal of the manganate concentration. These findings are in agreement with an equilibrium and a subsequent rate determining reaction according to the following scheme1, which has previously been proposed by Symons3  leading to the kinetic equation:
leading to the kinetic equation:  a—initial concentration of MnO4−, b—alkali concentration, x—concentration of MnO4=.
a—initial concentration of MnO4−, b—alkali concentration, x—concentration of MnO4=.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Landsberg, R., Geissler, W., and Müller, S., Z. Chemie, 1, 169 (1961). Landsberg, R., and Müller, S., Wiss. Z. T. H. Chemie Leuna-Merseburg, 3, 319 (1960–61).
Landsberg, R., and Thiele, R., Z. Phys. Chem., 221, 221 (1962).
Symons, M. C. R., J. Chem. Soc., 3956 (1953).
Symons, M. C. R., J. Chem. Soc., 3676 (1954).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
LANDSBERG, R., HECKNER, KH. An Isotopic Investigation of the Mechanism of Permanganate Decomposition in Alkaline Solution. Nature 201, 1123–1124 (1964). https://doi.org/10.1038/2011123a0
Issue Date:
DOI: https://doi.org/10.1038/2011123a0
This article is cited by
-
Oxidation of sodium (2-14C) acetate with alkaline permanganate
Journal of Radioanalytical Chemistry (1983)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.