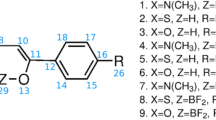
Abstract
ALTHOUGH the gaseous alkaline monofluoride molecules have been known for many years1, the stabilities of these molecules have not been accurately established. The Birge–Sponer extrapolations which Gaydon2 used to arrive at dissociation energies are in doubt because of the limited number of vibrational levels used and the fact that the data were often based on head measurements of bands of a weakly degraded system. Furthermore, these extrapolations are not expected to be normal for such molecules in which ionic forces make an appreciable contribution to the binding. This communication presents new results and theoretical calculations which indicate consistently high dissociation energies for all the alkaline earth monofluorides.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Herzberg, G., Molecular Spectra and Molecular Structure. 1. Spectra of Diatomic Molecules (D. Van Nostrand Co., Inc., New York, 1953).
Gaydon, A. G., Dissociation Energies (Chapman and Hall, Ltd., London, 1953).
Chupka, W. A., and Inghram, M. G., J. Chem. Phys., 21, 371 (1953); 59, 100 (1955).
Stull, D. R., and Sinke, G. C., Thermodynamic Properties of the Elements, Amer. Chem. Soc., Adv. Chem. Ser., No. 18 (1956).
JANAF Interim Thermochemical Tables, edit. by Stull, D. R. (Dow Chemical Co., Midland, Michigan, 1960).
Brewer, L., Somayajulu, G. R., and Brackett, E., Lawrence Rad. Lab Rep., UCRL-9840 (September 1961).
Kelley, K. K., U.S. Bur. Mines Bull., 584 (1960).
Kelley, K. K., and King, E. C., U.S. Bur. Mines Bull., 592 (1961).
Witt, W. P., and Barrow, R. F., Trans. Farad. Soc., 55, 730 (1959).
Hubbard, W. N. (private communication), ΔH0j 298 (MgF2,s) = −264.4 ± 0.6 kcal/mole.
a, Hammer, R. R., Messier, D. R., and Pask, J. A., Univ. Calif., Ceramic Lab., Ser. 18, issue 8 (1960). (b) Berkowitz, J., and Marquardt, J., J. Chem. Phys., 37, 1853 (1962).
Margrave, J. L., J. Phys. Chem., 58, 258 (1954).
Rittner, E. S., J. Chem. Phys., 19, 1030 (1951).
Pauling, L., and Wilson, E. B., Introduction to Quantum Mechanics, 204 (McGraw-Hill, New York, 1935).
Hirschfelder, J. O., Curtiss, C. F., and Bird, R. B., Molecular Theory of Gases and Liquids, 955 (John Wiley and Sons, Inc., New York, 1954).
Skinner, H. A., Trans. Farad. Soc., 45, 20 (1949).
Büchler, A., Klemperer, W., and Emslie, A. G., J. Chem. Phys., 36, 2499 (1962).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
BLUE, G., GREEN, J., EHLERT, T. et al. Dissociation Energies of the Alkaline Earth Monofluorides. Nature 199, 804–805 (1963). https://doi.org/10.1038/199804a0
Issue Date:
DOI: https://doi.org/10.1038/199804a0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.