Abstract
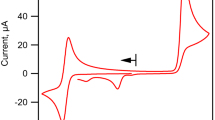
POLAROGRAPHIC behaviour and electrolytic reduction of nitrobenzene in a solution of 0.2 N sodium nitrate in dimethylformamide show that free radical anions of nitrobenzene are formed1,2. In this solution nitrobenzene is reduced in two polarographic waves, the first of which corresponds to the formation of the free radical anion C6H5–NO2−. These anions can be generated, in a special electrolytic vessel containing the solution, by electrolysis at a mercury pool cathode at a potential corresponding to the diffusion current of the first polarographic wave of nitrobenzene in dimethylformamide. The nitrobenzene anions generated were relatively stable in the absence of oxygen and were of a yellow-brown colour in solution. The anions formed gave a polarographic oxidation current (Fig. 1) corresponding to the formation of nitrobenzene: C6H5–NO2− – e→C6H5–NO2.
This is a preview of subscription content, access via your institution
Access options
Similar content being viewed by others
References
Kemula, W., and Sioda, R., Bull. Acad. Polon. Sci. (Serie Chimique), 10, No. 2, 107 (1962).
Kemula, W., and Sioda, R., Bull. Acad. Polon. Sci., (Serie Chimique) (in the press).
Paul, D. E., Lipkin, D., and Weissman, S. I., J. Amer. Chem. Soc., 78, 119 (1956).
Balk, P., Hoijtink, G. J., and Schreuvs, J. W. H., Rec. Trav. Chim., 76, 813 (1957).
Pointeau, R., and Favede, Mme., preprints of papers, 52–1, the fifth intern. symposium on free radicals, Uppsala (July, 1961).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
KEMULA, W., SIODA, R. Electrochemical Generation and Visible Spectrum of Nitrobenzene Free Radical Anion in Dimethylformamide. Nature 197, 588–589 (1963). https://doi.org/10.1038/197588a0
Issue Date:
DOI: https://doi.org/10.1038/197588a0
This article is cited by
-
Synthesis of nitrobenzene radical anion in a matrix and its IR absorption spectrum
Bulletin of the Academy of Sciences of the USSR Division of Chemical Science (1980)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.