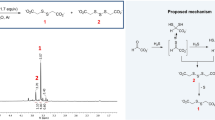
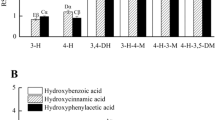
Abstract
THE hydroxylation of aromatic compounds in the animal body to form phenols is a well-established biochemical reaction (see review1) and has been known for nearly a hundred years. It was in 1867 that Schultzen and Naunyn2 first showed that benzene was converted to phenol in man and the dog. The reverse reaction, namely, dehydroxylation, is not well known. The first suggestion that such a reaction occurred in vivo was published in 1955 when Booth, Murray, DeEds and Jones3 noted an enhancement of the m-hydroxyphenylacetic acid output in the urine of rabbits that had been dosed with 3,4-di-hydroxyphenylacetic acid (homoprotocatechuic acid) or with the flavonoids, rutin and quercetin. It was suggested that the m-hydroxyphenylacetic acid arose by loss of the para-hydroxyl group of 3,4-dihydroxy-phenylacetic acid. The excretion of increased amounts of m-hydroxyphenyl acids has since been observed after the administration of 3,4-dihydroxycinnamic acid (caffeic acid) to man, the rabbit and the rat4,5 and after 3,4-dihydroxyphenylalanine (dopa) to the rabbit and the rat4. The ingestion of coffee leads to the excretion of increased amounts of m-hydroxyhippuric acid in human urine6, and this has been ascribed by Booth et al. 5 to the breakdown of chlorogenic acid, which is the quinic acid ester of caffeic acid, and which occurs in coffee beans to the extent of 5 per cent on a dry weight basis. According to Armstrong et al. 6,7, m-hydroxybenzoic, -phenylacetic, -phenylpropionic and probably -phenyllactic acids occur in human urine, but the origin of these meta-acids is obscure. However these acids could arise during metabolism by dehydroxylation of 3,4-dihydroxyphenyl acids (catechol acids). It is thus possible that dehydroxylation is a reaction of considerable metabolic significance.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Parke, D. V., and Williams, R. T., Ann. Rep. Chem. Soc., London, 55, 376 (1959).
Schultzen, O., and Naunyn, B., Arch. Anat. Physiol., 349 (1867).
Booth, A. N., Murray, C. W., DeEds, F., and Jones, F. T., Fed. Proc., 14, 321 (1955).
DeEds, F., Booth, A. N., and Jones, F. T., J. Biol. Chem., 225, 615 (1957).
Booth, A. N., Emerson, O. H., Jones, F. T., and DeEds, F., J. Biol. Chem., 229, 51 (1957).
Armstrong, M. D., Shaw, K. N. F., and Wall, P. E., J. Biol. Chem., 218, 293 (1956).
Armstrong, M. D., Wall, P. E., and Parker, V. G., J. Biol. Chem., 218, 921 (1956).
Takahashi, H., Kaihara, M., and Price, J. M., J. Biol. Chem., 223, 705 (1956).
Takahashi, H., and Price, J. M., J. Biol. Chem., 233, 150 (1958).
Goldstein, M., Friedhoff, A. J., and Simmons, C., Biochim. Biophys. Acta, 33, 372 (1959).
Williams, R. T., in “The Pharmacology of Plant Phenolics”, edit. by Fairbairn, J. N., 99 (Academic Press, London, 1959).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
SCHELINE, R., WILLIAMS, R. & WIT, J. Biological Dehydroxylation. Nature 188, 849–850 (1960). https://doi.org/10.1038/188849a0
Issue Date:
DOI: https://doi.org/10.1038/188849a0
This article is cited by
-
4-methylcatechol, a metabolite of homoprotocatechuic acid
Experientia (1967)
-
Untersuchungen über Physiologie und Pathophysiologie des sympathikoadrenalen Systems durch Bestimmung der Vanillin-Mandelsäure-Ausscheidung im Harn mit dünnschichtehromatographischer Technik
Archiv für Kreislaufforschung (1966)
-
Dehydroxylation of Caffeic Acid by Rat and Rabbit Cæcal Contents and Sheep Rumen Liquor
Nature (1963)
-
O-Methylation in Fish
Nature (1962)
-
m-Hydroxyphenylpropionic Acid, a Major Urinary Metabolite of (+)-Catechin in the Rat
Nature (1962)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.