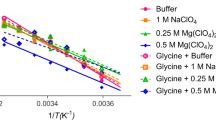
Abstract
KINETIC studies by Gutfreund and Sturtevant1 suggest that p-nitrophenyl acetate reacts with chymotrypsin rapidly at a pH greater than 6.5 to form monoacetyl chymotrypsin (AC–I) and that AC–I is then deacylated slowly. The kinetic observations are consistent with the esterification of a single serine hydroxyl of the enzyme2. In contrast, spectroscopic studies3 of the deacylation of monoacetyl chymotrypsin formed at pH 5.0 and isolated according to the procedure of Balls and Wood4, (AC–A), show that when AC–A is brought to pH 9.0, a rapid increase in absorption at 245 mµ occurs which is followed by slow decay of the absorption peak. Since both the absorption peak and its rate of decay appeared characteristic for N-acetyl imidazole, Dixon and Neurath3 suggested that the deacylation of monoacetyl chymotrypsin proceeds by a rapid intramolecular shift of the acetyl group from a serine oxygen to an imidazole nitrogen of the enzyme (AC–II), and that the rate-limiting step of the enzymatic reaction is the base-catalysed hydrolysis of N-acetyl imidazole. Recently, however, we have observed5 that AC–I and AC–Aare deacylated at different rates as measured by the liberation of p-nitrophenol from p-nitrophenyl acetate catalysed by AC–I and AC–A, and that AC–I is converted to AC–A under the conditions used in the isolation procedure4.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Gutfreund, H., and Sturtevant, J. M., Proc. U.S. Nat. Acad. Sci., 42, 719 (1956).
Cunningham, L. W., Science, 125, 1145 (1957).
Dixon, G. H., and Neurath, H., J. Amer. Chem. Soc., 79, 4558 (1957).
Balls, A. K., and Wood, H. N., J. Biol. Chem., 219, 245 (1956).
(a) Hess, G. P., and Marini, M. A., Abstracts, Fourth Internat. Congr. Biochemistry, 42 (Vienna, 1958). (b) Marini, M. A., and Hess, G. P., J. Amer. Chem. Soc., 81, 2594 (1959).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
MARINI, M., HESS, G. Reactivity and Interrelationship of Intermediates in the Hydrolysis of p-Nitrophenyl Acetate Catalysed by Chymotrypsin. Nature 184, 113–114 (1959). https://doi.org/10.1038/184113a0
Issue Date:
DOI: https://doi.org/10.1038/184113a0
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.