Abstract
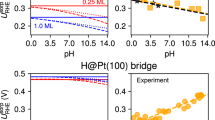
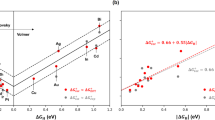
EXTENSIVE recent work1,2 on the hydrogen overpotentials at a large number of metal electrodes in aqueous solution allows a re-evaluation (see ref. 3) to be made of the relation between the hydrogen overpotential at various cathodes and the position of the cathode material in the periodic table. In the accompanying graph, the available values1,2 for the hydrogen overpotential at the intermediate current density of 10â3 amp./sq. cm. at various cathode materials, the surfaces of which have been prepared in approximately the same way, are shown in relation to the atomic number of the metals concerned, from which a periodic variation may clearly be discerned.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Hickling and Salt, Trans. Faraday Soc., 36, 1226 (1940).
Bockris, Trans. Faraday Soc., in the press.
Ellingham and Allmand, Trans. Faraday Soc., 19, 748 (1924). Partington, Chem. News, 129, 77 (1924).
For collection of modern values, see Klein and Lange, Z. EUclrochem., 43, 570 (1937).
Gurney, Proc. Roy. Soc., A, 134, 137 (1931).
Adam, "The Physics and Chemistry of Surfaces", 332 (Oxford, 1938).
Smits, Z. Eleetrochem., 30, 214 (1924). Erdy-Gruz and Volmer, Z. phys. Chem., 150, 203 (1930). Erdy-Gruz and Wick, Z. phys. Chem., 162, 53 (1932).
Frumkin, Z. phys. Chem., 160, 116 (1932).
Clark and Frölich, Z. Elektrochem., 31, 649 (1925).
Joffé Uspechi. Chem., 12, 438 (1943).
Erdy-Gruz and Volmer, Z. phys. Chem., 157, 165 (1931).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
BOCKRIS, J. Hydrogen Overpotential and the Thermionic Work Function. Nature 159, 539–540 (1947). https://doi.org/10.1038/159539b0
Issue Date:
DOI: https://doi.org/10.1038/159539b0
This article is cited by
-
Aspects of the theory of hydrogen overpotential
Experientia (1948)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.