Abstract
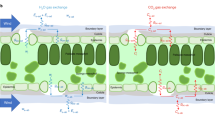
THE surface layer of wheat roots which are put in distilled water behaves like a membrane charged with hydrogen ions in a concentration of about 10-3 N. (pH = ca. 3)1. If a neutral salt is added to the water, the metallic cations are adsorbed in exchange for part of the hydrogen ions. As a consequence, the actual pH of the protoplasmic membrane increases; in a 10-3 N. solution of potassium chloride it is about pH = 6. If the solution has pH = 7 a potential difference of about 58 mv. occurs. The protoplasmic membrane is then negative relatively to the solution. This negative charge is the normal state of the absorption cells of the root tissue.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Lundegårdh, H., Biochem. Z., 298, 5 (1938).
Lundegårdh, H., Biochem. Z., 290, 104 (1937).
Lundegårdh, H., and Burström, Biochem. Z., 261, 235 (1933); 277, 223 (1935).
Krogh, A., NATURE, 140, 755 (1937).
Arens, K., Jahrb. Wiss. Botanik, 83, 561 (1936).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
LUNDEGÅRDH, H. An Electro-Chemical Theory of Salt Absorption and Respiration. Nature 143, 203–204 (1939). https://doi.org/10.1038/143203a0
Issue Date:
DOI: https://doi.org/10.1038/143203a0
This article is cited by
-
Metal nanoparticles as effective promotors for Maize production
Scientific Reports (2019)
-
Active ion uptake and maintenance of cation-anion balance: A critical examination of their role in regulating rhizosphere pH
Plant and Soil (1990)
-
A fuel cell model in biological energy conversion
Journal of Biological Physics (1985)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.