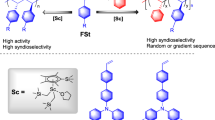
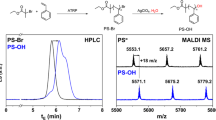
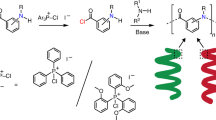
Abstract
INVESTIGATIONS of polymerization kinetics have often required rather cumbersome experimental methods, but Hammick and Langrish1 have recently applied a convenient bromination method for estimating cyclopentadiene and indene to a study of the polymerization rates of these compounds in carbon tetrachloride. We have been using, for some time, a very similar method to follow the kinetics of the polymerization of styrene, catalysed by anhydrous stannic chloride, in carbon tetrachloride and chloroform solutions at 25° C. The amount of residual monomeric styrene in the reaction mixture is determined, at known intervals of time, by quantitative bromine addition to the double bond, using an excess of bromine which is estimated by titration in the usual way. The accuracy of the analysis (better than 0.5 per cent) is not impaired by the presence of polystyrene ; but with polystyrene in the presence of stannic chloride, the excess bromine must be destroyed without delay, in order to avoid a slow attack of bromine on the polymer. The styrene disappearing during polymerization has been proved to be equivalent to the polymer formed by weighing the amount of polystyrene precipitated in methyl alcohol2. The polymers produced have molecular weights of the order 5,000 as determined by viscosity measurements.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Hammick, D. Ll., and Langrish, D., J. Chem. Soc., 797 (1937).
Schulz, G. V., and Husemann, E., Z. phys. Chem., B, 34, 187 (1936).
Skraup, S., and Freundlich, L., Annalen, 431, 243 (1923).
Gee, G., and Rideal, E. K., J. Chem. Soc., 772 (1937).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
WILLIAMS, G. Kinetics of Catalysed Polymerization of Styrene. Nature 140, 363–364 (1937). https://doi.org/10.1038/140363c0
Issue Date:
DOI: https://doi.org/10.1038/140363c0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.