Abstract
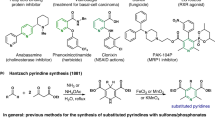
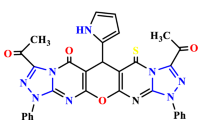
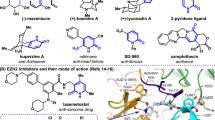
Structure-Activity Relationship (SAR) studies are fundamental to drug and agrochemical development, yet only a few synthetic strategies apply to the nitrogen heteroaromatics frequently encountered in small molecule candidates.1-3 Here, we present an alternative approach where we convert pyrimidine-containing compounds various other nitrogen heteroaromatics. Transforming pyrmidines into their corresponding N-arylpyrimidinium salts enables cleavage into a three-carbon iminoenamine building block, used for various heterocycle-forming reactions. This deconstruction-reconstruction sequence diversifies the initial pyrimidine core and enables access to various heterocycles, such as azoles.4 In effect, this approach allows heterocycle formation on complex molecules, resulting in analogs that would be challenging to obtain by other methods. We anticipate this deconstruction-reconstruction strategy will extend to other heterocycle classes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Rent or buy this article
Prices vary by article type
from$1.95
to$39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Author information
Authors and Affiliations
Corresponding authors
Supplementary information
Supplementary Information
Supplementary Information, including 19 Supplementary Figures, 4 Supplementary Tables, and additional references.
Rights and permissions
About this article
Cite this article
Uhlenbruck, B.J.H., Josephitis, C.M., de Lescure, L. et al. A deconstruction-reconstruction strategy for pyrimidine diversification. Nature (2024). https://doi.org/10.1038/s41586-024-07474-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41586-024-07474-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.