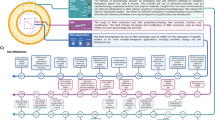
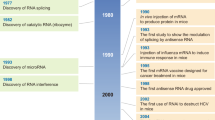
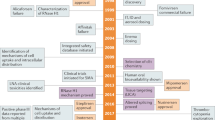
Abstract
RNA has sparked a revolution in modern medicine, with the potential to transform the way we treat diseases. Recent regulatory approvals, hundreds of new clinical trials, the emergence of CRISPR gene editing, and the effectiveness of mRNA vaccines in dramatic response to the COVID-19 pandemic have converged to create tremendous momentum and expectation. However, challenges with this relatively new class of drugs persist and require specialized knowledge and expertise to overcome. This Review explores shared strategies for developing RNA drug platforms, including layering technologies, addressing common biases and identifying gaps in understanding. It discusses the potential of RNA-based therapeutics to transform medicine, as well as the challenges associated with improving applicability, efficacy and safety profiles. Insights gained from RNA modalities such as antisense oligonucleotides (ASOs) and small interfering RNAs are used to identify important next steps for mRNA and gene editing technologies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Curreri, A., Sankholkar, D., Mitragotri, S. & Zhao, Z. RNA therapeutics in the clinic. Bioeng. Transl. Med. 8, e10374 (2023).
Anzalone, A. V., Koblan, L. W. & Liu, D. R. Genome editing with CRISPR-Cas nucleases, base editors, transposases and prime editors. Nat. Biotechnol. 38, 824–844 (2020).
Xie, W., Chen, B. & Wong, J. Evolution of the market for mRNA technology. Nat. Rev. Drug Discov. 20, 735–736 (2021).
Hogan, M. J. & Pardi, N. mRNA vaccines in the COVID-19 pandemic and beyond. Annu. Rev. Med. 73, 17–39 (2022).
Warne, N. et al. Delivering 3 billion doses of Comirnaty in 2021. Nat. Biotechnol. 41, 183–188 (2023). This article provides first-hand account of the technical and operational feats required to deliver large-scale production of a COVID-19 mRNA vaccine during the pandemic.
Polack, F. P. et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N. Engl. J. Med. 383, 2603–2615 (2020). This article is a landmark publication for mRNA vaccines, showing 95% protection against COVID-19.
Thomas, S. J. et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine through 6 months. N. Engl. J. Med. 385, 1761–1773 (2021).
Baden, L. R. et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 384, 403–416 (2021). This article describes the success of a second COVID-19 vaccine, solidifying mRNA as a game-changing technology for addressing the pandemic.
Jones, C. H. et al. Breaking the mold with RNA-a “RNAissance” of life science. NPJ Genom. Med. 9, 2 (2024).
Griffin, R. Pfizer needs to prove it’s ready to move on from Covid-19. Bloomberg News https://www.bloomberg.com/news/features/2022-10-04/pfizer-s-pfe-future-relies-on-mrna-scientists-are-skeptical (2022).
Orbital Therapeutics. Orbital Therapeutics secures $270 million in series a financing to unleash full potential of RNA medicines. businesswire https://www.businesswire.com/news/home/20230426005102/en/Orbital-Therapeutics-Secures-270-Million-in-Series-A-Financing-to-Unleash-Full-Potential-of-RNA-Medicines (2023).
Chroma Medicine. Chroma Medicine launches with $125M in financing to deliver on the promise of epigenetic editing. PR Newswire https://www.prnewswire.com/news-releases/chroma-medicine-launches-with-125m-in-financing-to-deliver-on-the-promise-of-epigenetic-editing-301426248.html (2021).
ReNAgade Therapeutics. ReNAgade Therapeutics launches with over $300 million in series A financing to unlock the limitless potential of RNA medicine. MarketWatch https://www.marketwatch.com/press-release/renagade-therapeutics-launches-with-over-300-million-in-series-a-financing-to-unlock-the-limitless-potential-of-rna-medicine-2023-05-23 (2023).
Kozlowski, K. New UM Center to take medicine to personalized level. The Detroit News https://www.detroitnews.com/story/life/wellness/2016/03/20/center-rna-biomedicine-university-michigangenomeprostatemips/82060600/ (2016).
Monnet, R. $165-million for McGill University’s world-leading inclusive genomics and RNA research program. McGill University https://www.mcgill.ca/gci/article/165-million-mcgill-universitys-world-leading-inclusive-genomics-and-rna-research-program (2023).
University at Albany. UAlbany research at the RNA Institute, NYS Mesonet receives $2 million federal boost. University at Albany https://www.albany.edu/news-center/news/2022-ualbany-research-rna-institute-nys-mesonet-receives-2-million-federal-boost (2022).
Houston Methodist Academic Institute. Annual Report 2021: Expanding the RNAcore to encompass the entire cycle of translation. issuu https://issuu.com/instituteforacademicmedicine/docs/hmai_annual_report_2021/s/16433340 (2022).
Hait, W. N. & Stoffels, P. A primer for academic entrepreneurs on academic-industrial partnerships. Nat. Commun. 12, 5778 (2021).
Chitale, S., Lawler, C. & Klausner, A. So you want to start a biotech company. Nat. Biotechnol. 40, 296–300 (2022).
Damase, T. R. et al. The limitless future of RNA therapeutics. Front. Bioeng. Biotechnol. 9, 628137 (2021).
Gleeson, J. G. et al. Personalized antisense oligonucleotides “for free, for life” — the n-Lorem Foundation. Nat. Med. 29, 1302–1303 (2023).
Fernando, K. et al. Achieving end-to-end success in the clinic: Pfizer’s learnings on R&D productivity. Drug Discov. Today 27, 697–704 (2022).
Aimo, A. et al. RNA-targeting and gene editing therapies for transthyretin amyloidosis. Nat. Rev. Cardiol. 19, 655–667 (2022).
Walsh, G. & Walsh, E. Biopharmaceutical benchmarks 2022. Nat. Biotechnol. 40, 1722–1760 (2022).
Fire, A. et al. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391, 806–811 (1998). This article describes a Nobel Prize winning primary work on the discovery of siRNA.
Liu, J. K. H. The history of monoclonal antibody development — progress, remaining challenges and future innovations. Ann. Med. Surg. 3, 113–116 (2014).
Eisenstein, M. Pharma’s roller-coaster relationship with RNA therapies. Nature 574, S4–S6 (2019).
Mathieu, E. et al. Coronavirus pandemic (COVID-19). Our World in Data https://ourworldindata.org/coronavirus (2020).
Dolgin, E. Self-copying RNA vaccine wins first full approval: what’s next? Nature 624, 236–237 (2023).
Winokur, P. et al. Bivalent omicron BA.1-adapted BNT162b2 booster in adults older than 55 years. N. Engl. J. Med. 388, 214–227 (2023).
Chalkias, S. et al. A bivalent omicron-containing booster vaccine against Covid-19. N. Engl. J. Med. 387, 1279–1291 (2022).
Pecetta, S. & Rappuoli, R. mRNA, the beginning of a new influenza vaccine game. Proc. Natl Acad. Sci. USA 119, e2217533119 (2022).
Pardi, N., Hogan, M. J., Porter, F. W. & Weissman, D. mRNA vaccines — a new era in vaccinology. Nat. Rev. Drug Discov. 17, 261–279 (2018).
RNA Therapy Beacon (Hanson Wade Group, 2023); https://hansonwade.com/rna-therapy-beacon/.
Center for Drug Evaluation & Research. FDA approves treatment of amyotrophic lateral sclerosis associated with a mutation in the SOD1 gene. US Food and Drug Administration https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-treatment-amyotrophic-lateral-sclerosis-associated-mutation-sod1-gene (2023).
Aoki, Y. & Wood, M. J. A. Emerging oligonucleotide therapeutics for rare neuromuscular diseases. J. Neuromuscul. Dis. 8, 869–884 (2021).
Armengol, V. D. et al. Life-saving treatments for spinal muscular atrophy: global access and availability. Neurol. Clin. Pract. 14, e200224 (2024).
Egli, M. & Manoharan, M. Chemistry, structure and function of approved oligonucleotide therapeutics. Nucleic Acids Res. 51, 2529–2573 (2023). This is a thorough, well-organized and accessible review article on oligonucleotide chemistries.
Woodcock, J. & Marks, P. Drug regulation in the era of individualized therapies. N. Engl. J. Med. 381, 1678–1680 (2019).
Investor Relations Alnylam Pharmaceuticals, Inc. Alnylam and Regeneron report positive interim phase 1 clinical data on ALN-APP, an investigational RNAi therapy. Alnylam Pharmaceuticals, Inc. https://investors.alnylam.com/press-release? (2023).
Lim, G. B. Novel siRNA reduces plasma lipoprotein(a) levels. Nat. Rev. Cardiol. 19, 147 (2022).
Zhang, M. M., Bahal, R., Rasmussen, T. P., Manautou, J. E. & Zhong, X.-B. The growth of siRNA-based therapeutics: updated clinical studies. Biochem. Pharmacol. 189, 114432 (2021).
Beck, J. D. et al. mRNA therapeutics in cancer immunotherapy. Mol. Cancer 20, 69 (2021).
Vitale, G. Moderna/Merck cancer vaccine shows promise in trials. Chemical & Engineering News https://cen.acs.org/pharmaceuticals/vaccines/ModernaMerck-cancer-vaccine-shows-promise/100/web/2022/12 (2022).
Gillmore, J. D. et al. CRISPR-Cas9 in vivo gene editing for transthyretin amyloidosis. N. Engl. J. Med. 385, 493–502 (2021). To our knowledge, this is the first published clinical study report for in vivo CRISPR gene editing.
Cross, R. Intellia’s second CRISPR gene editing therapy reduces swelling attacks by 95% in hereditary angioedema. Endpoints News https://endpts.com/intellias-second-crispr-gene-editing-therapy-reduces-swelling-attacks-by-95-in-hereditary-angioedema/ (2023).
Naddaf, M. First trial of “base editing” in humans lowers cholesterol — but raises safety concerns. Nature 623, 671–672 (2023).
Crooke, S. T., Liang, X.-H., Baker, B. F. & Crooke, R. M. Antisense technology: a review. J. Biol. Chem. 296, 100416 (2021). This review is a tour de force of the deep mechanistic knowledge acquired over decades of ASO platform research.
Roberts, T. C., Langer, R. & Wood, M. J. A. Advances in oligonucleotide drug delivery. Nat. Rev. Drug Discov. 623, 673–694 (2020).
Kawamoto, Y., Wu, Y., Takahashi, Y. & Takakura, Y. Development of nucleic acid medicines based on chemical technology. Adv. Drug Deliv. Rev. 199, 114872 (2023).
Aliahmad, P., Miyake-Stoner, S. J., Geall, A. J. & Wang, N. S. Next generation self-replicating RNA vectors for vaccines and immunotherapies. Cancer Gene Ther. 30, 785–793 (2022).
Wesselhoeft, R. A., Kowalski, P. S. & Anderson, D. G. Engineering circular RNA for potent and stable translation in eukaryotic cells. Nat. Commun. 9, 2629 (2018). This study describes pioneering work on the production and use of circRNA for longer-lived protein expression in transfected cells compared with linear mRNA.
Hardee, G. E., Tillman, L. G. & Geary, R. S. in Antisense Drug Technolology 2nd edn, Ch. 8, 217–236 (Routledge, 2007).
Levin, A. A., Rosie, Z. Y. & Geary, R. S. in Antisense Drug Technology 2nd edn, Ch. 7, 183–216 (Routledge, 2007).
Iversen, P. L. in Antisense Drug Technology 2nd edn, Ch. 20, 565–582 (Routledge, 2007).
Crooke, S. T., Wang, S., Vickers, T. A., Shen, W. & Liang, X.-H. Cellular uptake and trafficking of antisense oligonucleotides. Nat. Biotechnol. 35, 230–237 (2017).
Kim, J. et al. Patient-customized oligonucleotide therapy for a rare genetic disease. N. Engl. J. Med. 381, 1644–1652 (2019). This study provides clinical proof-of-concept for hyper-personalized medicine using ASOs.
Rittig, S. M. et al. Intradermal vaccinations with RNA coding for TAA generate CD8+ and CD4+ immune responses and induce clinical benefit in vaccinated patients. Mol. Ther. 19, 990–999 (2011).
Weide, B. et al. Results of the first phase I/II clinical vaccination trial with direct injection of mRNA. J. Immunother. 31, 180–188 (2008).
Selmi, A. et al. Uptake of synthetic naked RNA by skin-resident dendritic cells via macropinocytosis allows antigen expression and induction of T-cell responses in mice. Cancer Immunol. Immunother. 65, 1075–1083 (2016).
Wolff, J. A. et al. Direct gene transfer into mouse muscle in vivo. Science 247, 1465–1468 (1990). This study demonstrates that intramuscular injection into mice of both lipid-formulated or ‘naked’ mRNA results in protein expression.
Probst, J. et al. Spontaneous cellular uptake of exogenous messenger RNA in vivo is nucleic acid-specific, saturable and ion dependent. Gene Ther. 14, 1175–1180 (2007).
Ramachandran, S., Satapathy, S. R. & Dutta, T. Delivery strategies for mRNA vaccines. Pharm. Med. 36, 11–20 (2022).
Anttila, V. et al. Direct intramyocardial injection of VEGF mRNA in patients undergoing coronary artery bypass grafting. Mol. Ther. 31, 866–874 (2023).
Diken, M. et al. Selective uptake of naked vaccine RNA by dendritic cells is driven by macropinocytosis and abrogated upon DC maturation. Gene Ther. 18, 702–708 (2011).
Prakash, T. P. et al. Identification of metabolically stable 5′-phosphate analogs that support single-stranded siRNA activity. Nucleic Acids Res. 43, 2993–3011 (2015).
Mitchell, M. J. et al. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 20, 101–124 (2021).
Khorev, O., Stokmaier, D., Schwardt, O., Cutting, B. & Ernst, B. Trivalent, Gal/GalNAc-containing ligands designed for the asialoglycoprotein receptor. Bioorg. Med. Chem. 16, 5216–5231 (2008).
Tanowitz, M. et al. Asialoglycoprotein receptor 1 mediates productive uptake of N-acetylgalactosamine-conjugated and unconjugated phosphorothioate antisense oligonucleotides into liver hepatocytes. Nucleic Acids Res. 45, 12388–12400 (2017).
Nair, J. K. et al. Multivalent N-acetylgalactosamine-conjugated siRNA localizes in hepatocytes and elicits robust RNAi-mediated gene silencing. J. Am. Chem. Soc. 136, 16958–16961 (2014).
Akinc, A. et al. Targeted delivery of RNAi therapeutics with endogenous and exogenous ligand-based mechanisms. Mol. Ther. 18, 1357–1364 (2010). This study elucidates the roles of ApoE and LDLR in LNP uptake, and even more significantly, it demonstrates GalNAc-conjugated siRNA delivery.
Francia, V., Schiffelers, R. M., Cullis, P. R. & Witzigmann, D. The biomolecular corona of lipid nanoparticles for gene therapy. Bioconjug. Chem. 31, 2046–2059 (2020).
Paunovska, K. et al. The extent to which lipid nanoparticles require apolipoprotein E and low-density lipoprotein receptor for delivery changes with ionizable lipid structure. Nano Lett. 22, 10025–10033 (2022).
Dilliard, S. A., Cheng, Q. & Siegwart, D. J. On the mechanism of tissue-specific mRNA delivery by selective organ targeting nanoparticles. Proc. Natl Acad. Sci. USA 118, e2109256118 (2021). This seminal study provides proof-of-concept for passive targeted LNPs wherein supplemental LNP formulation components influence protein corona formation, thereby changing biodistribution to the liver, lung and spleen.
Cheng, Q. et al. Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR-Cas gene editing. Nat. Nanotechnol. 15, 313–320 (2020).
Rurik, J. G. et al. CAR T cells produced in vivo to treat cardiac injury. Science 375, 91–96 (2022). This article reports pioneering work for in vivo cellular reprogramming using an anti-CD5 mAb-conjugated LNP to generate antifibrotic CAR T cells in situ following intravenous administration of a formulated CAR-encoding mRNA.
Parhiz, H. et al. PECAM-1 directed re-targeting of exogenous mRNA providing two orders of magnitude enhancement of vascular delivery and expression in lungs independent of apolipoprotein E-mediated uptake. J. Control. Rel. 291, 106–115 (2018).
Pascual-Gilabert, M., Artero, R. & López-Castel, A. The myotonic dystrophy type 1 drug development pipeline: 2022 edition. Drug Discov. Today 28, 103489 (2023).
Akinc, A. et al. The Onpattro story and the clinical translation of nanomedicines containing nucleic acid-based drugs. Nat. Nanotechnol. 14, 1084–1087 (2019).
Thi, T. T. H. et al. Lipid-based nanoparticles in the clinic and clinical trials: from cancer nanomedicine to COVID-19 vaccines. Vaccines 9, 354 (2021).
Paunovska, K., Loughrey, D. & Dahlman, J. E. Drug delivery systems for RNA therapeutics. Nat. Rev. Genet. 23, 265–280 (2022).
Semple, S. C. et al. Efficient encapsulation of antisense oligonucleotides in lipid vesicles using ionizable aminolipids: formation of novel small multilamellar vesicle structures. Biochim. Biophys. Acta 1510, 152–166 (2001).
Semple, S. C. et al. Rational design of cationic lipids for siRNA delivery. Nat. Biotechnol. 28, 172–176 (2010). This is an early breakthrough study for LNPs showing improved siRNA knockdown of a tool gene in livers of rodents and NHPs.
Jayaraman, M. et al. Maximizing the potency of siRNA lipid nanoparticles for hepatic gene silencing in vivo. Angew. Chem. Int. Ed. 51, 8529–8533 (2012).
Akinc, A. et al. A combinatorial library of lipid-like materials for delivery of RNAi therapeutics. Nat. Biotechnol. 26, 561–569 (2008).
Love, K. T. et al. Lipid-like materials for low-dose, in vivo gene silencing. Proc. Natl Acad. Sci. USA 107, 1864–1869 (2010).
Gilleron, J. et al. Image-based analysis of lipid nanoparticle-mediated siRNA delivery, intracellular trafficking and endosomal escape. Nat. Biotechnol. 31, 638–646 (2013). This article provides a good example of the meticulous and challenging aspects of studying endosomal release of RNA payloads.
Sahay, G. et al. Efficiency of siRNA delivery by lipid nanoparticles is limited by endocytic recycling. Nat. Biotechnol. 31, 653–658 (2013).
Dowdy, S. F. Endosomal escape of RNA therapeutics: how do we solve this rate-limiting problem? RNA 29, 396–401 (2023).
Geary, R. S. Antisense oligonucleotide pharmacokinetics and metabolism. Expert Opin. Drug Metab. Toxicol. 5, 381–391 (2009).
Prakash, T. P. et al. Positional effect of chemical modifications on short interference RNA activity in mammalian cells. J. Med. Chem. 48, 4247–4253 (2005).
Manoharan, M. et al. Unique gene-silencing and structural properties of 2′-fluoro-modified siRNAs. Angew. Chem. Int. Ed. 50, 2284–2288 (2011).
Brown, C. R. et al. Investigating the pharmacodynamic durability of GalNAc-siRNA conjugates. Nucleic Acids Res. 48, 11827–11844 (2020).
Janas, M. M. et al. Safety evaluation of 2′-deoxy-2′-fluoro nucleotides in GalNAc-siRNA conjugates. Nucleic Acids Res. 47, 3306–3320 (2019).
Parmar, R. G. et al. Facile synthesis, geometry, and 2′-substituent-dependent in vivo activity of 5′-(E)- and 5′-(Z)-vinylphosphonate-modified siRNA conjugates. J. Med. Chem. 61, 734–744 (2018).
Andries, O. et al. N1-Methylpseudouridine-incorporated mRNA outperforms pseudouridine-incorporated mRNA by providing enhanced protein expression and reduced immunogenicity in mammalian cell lines and mice. J. Control. Rel. 217, 337–344 (2015).
Mauger, D. M. et al. mRNA structure regulates protein expression through changes in functional half-life. Proc. Natl Acad. Sci. USA 116, 24075–24083 (2019).
Leppek, K. et al. Combinatorial optimization of mRNA structure, stability, and translation for RNA-based therapeutics. Nat. Commun. 13, 1536 (2022).
DeRosa, F. et al. Therapeutic efficacy in a hemophilia B model using a biosynthetic mRNA liver depot system. Gene Ther. 23, 699–707 (2016).
August, A. et al. A phase 1 trial of lipid-encapsulated mRNA encoding a monoclonal antibody with neutralizing activity against chikungunya virus. Nat. Med. 27, 2224–2233 (2021). This article is one of few published clinical studies reporting measured levels of protein expression and pharmacokinetics of an infused mRNA-LNP.
Sahin, U., Karikó, K. & Türeci, Ö. mRNA-based therapeutics-developing a new class of drugs. Nat. Rev. Drug Discov. 13, 759–780 (2014).
Roseman, D. S. et al. G6PC mRNA therapy positively regulates fasting blood glucose and decreases liver abnormalities in a mouse model of glycogen storage disease 1a. Mol. Ther. 26, 814–821 (2018).
Chen, C.-K. et al. Structured elements drive extensive circular RNA translation. Mol. Cell 81, 4300–4318.e13 (2021).
Enuka, Y. et al. Circular RNAs are long-lived and display only minimal early alterations in response to a growth factor. Nucleic Acids Res. 44, 1370–1383 (2016).
Hansen, T. B. et al. Natural RNA circles function as efficient microRNA sponges. Nature 495, 384–388 (2013).
Legnini, I. et al. Circ-ZNF609 is a circular RNA that can be translated and functions in myogenesis. Mol. Cell 66, 22–37.e9 (2017).
Pamudurti, N. R. et al. Translation of circRNAs. Mol. Cell 66, 9–21.e7 (2017).
Yang, Y. et al. Extensive translation of circular RNAs driven by N6-methyladenosine. Cell Res. 27, 626–641 (2017).
Wesselhoeft, R. A. et al. RNA circularization diminishes immunogenicity and can extend translation duration in vivo. Mol. Cell 74, 508–520.e4 (2019).
Geall, A. J. et al. Nonviral delivery of self-amplifying RNA vaccines. Proc. Natl Acad. Sci. USA 109, 14604–14609 (2012). This article reports seminal saRNA vaccine proof-of-concept work demonstrating immunogenicity and protection against RSV in a cotton rat model.
Oda, Y. et al. Immunogenicity and safety of a booster dose of a self-amplifying RNA COVID-19 vaccine (ARCT-154) versus BNT162b2 mRNA COVID-19 vaccine: a double-blind, multicentre, randomised, controlled, phase 3, non-inferiority trial. Lancet Infect. Dis. 24, 351–360 (2023).
McKay, P. F. et al. Self-amplifying RNA SARS-CoV-2 lipid nanoparticle vaccine candidate induces high neutralizing antibody titers in mice. Nat. Commun. 11, 3523 (2020).
Vogel, A. B. et al. Self-amplifying RNA vaccines give equivalent protection against influenza to mRNA vaccines but at much lower doses. Mol. Ther. 26, 446–455 (2018).
de Alwis, R. et al. A single dose of self-transcribing and replicating RNA-based SARS-CoV-2 vaccine produces protective adaptive immunity in mice. Mol. Ther. 29, 1970–1983 (2021).
Zhang, X. et al. Patisiran pharmacokinetics, pharmacodynamics, and exposure-response analyses in the phase 3 APOLLO trial in patients with hereditary transthyretin-mediated (hATTR) amyloidosis. J. Clin. Pharmacol. 60, 37–49 (2020).
Maier, M. A. et al. Biodegradable lipids enabling rapidly eliminated lipid nanoparticles for systemic delivery of RNAi therapeutics. Mol. Ther. 21, 1570–1578 (2013).
Sabnis, S. et al. A novel amino lipid series for mRNA delivery: improved endosomal escape and sustained pharmacology and safety in non-human primates. Mol. Ther. 26, 1509–1519 (2018).
Walsh, E. E. et al. Safety and immunogenicity of two RNA-based covid-19 vaccine candidates. N. Engl. J. Med. 383, 2439–2450 (2020).
Adams, D. et al. Long-term safety and efficacy of patisiran for hereditary transthyretin-mediated amyloidosis with polyneuropathy: 12-month results of an open-label extension study. Lancet Neurol. 20, 49–59 (2021).
Tahtinen, S. et al. IL-1 and IL-1ra are key regulators of the inflammatory response to RNA vaccines. Nat. Immunol. 23, 532–542 (2022). In this article, the authors identify a plausible immunological mechanism for increased sensitivity of humans to mRNA-LNP compared with preclinical species.
Robbins, M., Judge, A. & MacLachlan, I. siRNA and innate immunity. Oligonucleotides 19, 89–102 (2009).
Parhiz, H. et al. Added to pre-existing inflammation, mRNA-lipid nanoparticles induce inflammation exacerbation (IE). J. Control. Rel. 344, 50–61 (2021).
Swayze, E. E. & Bhat, B. in Antisense Drug Technology 2nd edn, Ch. 6, 143–182 (Routledge, 2007).
Morrissey, D. V. et al. Potent and persistent in vivo anti-HBV activity of chemically modified siRNAs. Nat. Biotechnol. 23, 1002–1007 (2005).
Karikó, K., Buckstein, M., Ni, H. & Weissman, D. Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity 23, 165–175 (2005). This article reports transformative work, although less appreciated at the time, on chemically modified mRNA and recognition by pattern recognition receptors.
Abbas, Y. M. et al. Structure of human IFIT1 with capped RNA reveals adaptable mRNA binding and mechanisms for sensing N1 and N2 ribose 2′-O methylations. Proc. Natl Acad. Sci. USA 114, E2106–E2115 (2017).
Minnaert, A.-K. et al. Strategies for controlling the innate immune activity of conventional and self-amplifying mRNA therapeutics: getting the message across. Adv. Drug Deliv. Rev. 176, 113900 (2021).
Szebeni, J. Complement activation-related pseudoallergy: a stress reaction in blood triggered by nanomedicines and biologicals. Mol. Immunol. 61, 163–173 (2014).
Coelho, T. et al. Safety and efficacy of RNAi therapy for transthyretin amyloidosis. N. Engl. J. Med. 369, 819–829 (2013).
Crooke, S. T., Seth, P. P., Vickers, T. A. & Liang, X.-H. The interaction of phosphorothioate-containing RNA targeted drugs with proteins is a critical determinant of the therapeutic effects of these agents. J. Am. Chem. Soc. 142, 14754–14771 (2020).
Chi, X., Gatti, P. & Papoian, T. Safety of antisense oligonucleotide and siRNA-based therapeutics. Drug Discov. Today 22, 823–833 (2017).
Swayze, E. E. et al. Antisense oligonucleotides containing locked nucleic acid improve potency but cause significant hepatotoxicity in animals. Nucleic Acids Res. 35, 687–700 (2007).
Jackson, A. L. et al. Widespread siRNA “off-target” transcript silencing mediated by seed region sequence complementarity. RNA 12, 1179–1187 (2006).
Janas, M. M. et al. Selection of GalNAc-conjugated siRNAs with limited off-target-driven rat hepatotoxicity. Nat. Commun. 9, 723 (2018).
Schlegel, M. K. et al. Overcoming GNA/RNA base-pairing limitations using isonucleotides improves the pharmacodynamic activity of ESC+ GalNAc-siRNAs. Nucleic Acids Res. 49, 10851–10867 (2021).
Sedic, M. et al. Safety evaluation of lipid nanoparticle-formulated modified mRNA in the Sprague-Dawley rat and cynomolgus monkey. Vet. Pathol. 55, 341–354 (2018).
Loughrey, D. & Dahlman, J. E. Non-liver mRNA delivery. Acc. Chem. Res. 55, 13–23 (2022).
Maraganore, J. Reflections on Alnylam. Nat. Biotechnol. 40, 641–650 (2022). This article provides an insightful retrospective on the business and science behind Alnylam’s platform build.
Tseng, H. F. et al. mRNA-1273 bivalent (original and Omicron) COVID-19 vaccine effectiveness against COVID-19 outcomes in the United States. Nat. Commun. 14, 5851 (2023).
Zou, J. et al. Neutralization of BA.4-BA.5, BA.4.6, BA.2.75.2, BQ.1.1, and XBB.1 with bivalent vaccine. N. Engl. J. Med. 388, 854–857 (2023).
Stewart-Jones, G. B. E. et al. Domain-based mRNA vaccines encoding spike protein N-terminal and receptor binding domains confer protection against SARS-CoV-2. Sci. Transl. Med. 15, eadf4100 (2023).
Brown, K. M. et al. Expanding RNAi therapeutics to extrahepatic tissues with lipophilic conjugates. Nat. Biotechnol. 40, 1500–1508 (2022).
Obici, L. et al. Quality of life outcomes in APOLLO, the phase 3 trial of the RNAi therapeutic patisiran in patients with hereditary transthyretin-mediated amyloidosis. Amyloid 27, 153–162 (2020).
Adams, D. et al. Patisiran, an RNAi therapeutic, for hereditary transthyretin amyloidosis. N. Engl. J. Med. 379, 11–21 (2018).
Butler, J. S. et al. Preclinical evaluation of RNAi as a treatment for transthyretin-mediated amyloidosis. Amyloid 23, 109–118 (2016).
Sutherland, J. E. et al. Nonclinical safety profile of revusiran, a 1st-generation GalNAc-siRNA conjugate for treatment of hereditary transthyretin-mediated amyloidosis. Nucleic Acid Ther. 30, 33–49 (2020).
Judge, D. P. et al. Phase 3 multicenter study of revusiran in patients with hereditary transthyretin-mediated (hATTR) amyloidosis with cardiomyopathy (ENDEAVOUR). Cardiovasc. Drugs Ther. 34, 357–370 (2020).
Hawkins, P. N. et al. Evolving landscape in the management of transthyretin amyloidosis. Ann. Med. 47, 625–638 (2015).
Adams, D. et al. Efficacy and safety of vutrisiran for patients with hereditary transthyretin-mediated amyloidosis with polyneuropathy: a randomized clinical trial. Amyloid 30, 18–26 (2023).
Investor Relations Alnylam Pharmaceuticals, Inc. Alnylam continues scientific leadership in advancement of RNAi therapeutics at the 17th Annual Meeting of the Oligonucleotides Therapeutics Society. Alnylam Pharmaceuticals, Inc. https://investors.alnylam.com/press-release?id=26011 (2021).
Keam, S. J. Inotersen: first global approval. Drugs 78, 1371–1376 (2018).
Ackermann, E. J. et al. Suppressing transthyretin production in mice, monkeys and humans using 2nd-generation antisense oligonucleotides. Amyloid 23, 148–157 (2016).
Benson, M. D. et al. Inotersen treatment for patients with hereditary transthyretin amyloidosis. N. Engl. J. Med. 379, 22–31 (2018).
Crooke, S. T. et al. The effects of 2′-O-methoxyethyl containing antisense oligonucleotides on platelets in human clinical trials. Nucleic Acid Ther. 27, 121–129 (2017).
Slingsby, M. H. L. et al. Sequence-specific 2′-O-methoxyethyl antisense oligonucleotides activate human platelets through glycoprotein VI, triggering formation of platelet-leukocyte aggregates. Haematologica 107, 519–531 (2022).
Zaslavsky, A. et al. Antisense oligonucleotides and nucleic acids generate hypersensitive platelets. Thromb. Res. 200, 64–71 (2021).
Flierl, U. et al. Phosphorothioate backbone modifications of nucleotide-based drugs are potent platelet activators. J. Exp. Med. 212, 129–137 (2015).
Engelhardt, J. A. et al. Scientific and regulatory policy committee points-to-consider paper*: drug-induced vascular injury associated with nonsmall molecule therapeutics in preclinical development: part 2. antisense oligonucleotides. Toxicol. Pathol. 43, 935–944 (2015).
Shen, L. et al. Mechanistic understanding for the greater sensitivity of monkeys to antisense oligonucleotide-mediated complement activation compared with humans. J. Pharmacol. Exp. Ther. 351, 709–717 (2014).
Frazier, K. S. et al. Species-specific inflammatory responses as a primary component for the development of glomerular lesions in mice and monkeys following chronic administration of a second-generation antisense oligonucleotide. Toxicol. Pathol. 42, 923–935 (2014).
US Food and Drug Administration. WAINUA™ (eplontersen) injection, for subcutaneous use initial U.S. approval. US Food and Drug Administration https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/217388s000lbl.pdf (2023).
Ionis Pharmaceuticals, Inc. Ionis announces FDA acceptance of new drug application for eplontersen for the treatment of hereditary transthyretin-mediated amyloid polyneuropathy (ATTRv-PN). PR Newswire https://www.prnewswire.com/news-releases/ionis-announces-fda-acceptance-of-new-drug-application-for-eplontersen-for-the-treatment-of-hereditary-transthyretin-mediated-amyloid-polyneuropathy-attrv-pn-301764027.html (2023).
Finn, J. D. et al. A single administration of CRISPR/Cas9 lipid nanoparticles achieves robust and persistent in vivo genome editing. Cell Rep. 22, 2227–2235 (2018). To our knowledge, this is the first paper to demonstrate that a transient mRNA-LNP can be used for stable in vivo gene editing of a mouse.
Intellia Therapeutics. Intellia and Regeneron present updated interim data from phase 1 study of CRISPR-based NTLA-2001 for the treatment of transthyretin (ATTR) amyloidosis demonstrating that deep serum TTR reductions remained durable after a single dose. Intellia Therapeutics https://ir.intelliatx.com/news-releases/news-release-details/intellia-and-regeneron-present-updated-interim-data-phase-1 (2022).
Intellia Therapeutics. Intellia presents updated interim data from the cardiomyopathy arm of ongoing phase 1 study of NTLA-2001, an investigational CRISPR therapy for the treatment of transthyretin (ATTR) amyloidosis at the American Heart Association scientific sessions 2022. Intellia Therapeutics https://ir.intelliatx.com/news-releases/news-release-details/intellia-presents-updated-interim-data-cardiomyopathy-arm (2022).
Intellia Therapeutics. Intellia Therapeutics announces new positive clinical data from phase 1 study of NTLA-2002, an investigational in vivo CRISPR genome editing treatment for hereditary angioedema (HAE). Intellia Therapeutics https://ir.intelliatx.com/news-releases/news-release-details/intellia-therapeutics-announces-new-positive-clinical-data-phase (2022).
Garrelfs, S. F. et al. Lumasiran, an RNAi therapeutic for primary hyperoxaluria type 1. N. Engl. J. Med. 384, 1216–1226 (2021).
Havens, M. A. & Hastings, M. L. Splice-switching antisense oligonucleotides as therapeutic drugs. Nucleic Acids Res. 44, 6549–6563 (2016).
Booth, B. J. et al. RNA editing: expanding the potential of RNA therapeutics. Mol. Ther. 31, 1533–1549 (2023).
Perez-Garcia, C. G. et al. Development of an mRNA replacement therapy for phenylketonuria. Mol. Ther. Nucleic Acids 28, 87–98 (2022).
Jericó, D. et al. mRNA-based therapy in a rabbit model of variegate porphyria offers new insights into the pathogenesis of acute attacks. Mol. Ther. Nucleic Acids 25, 207–219 (2021).
An, D. et al. Systemic messenger RNA therapy as a treatment for methylmalonic acidemia. Cell Rep. 24, 2520 (2018).
Apgar, J. F. et al. Quantitative systems pharmacology model of hUGT1A1-modRNA encoding for the UGT1A1 enzyme to treat Crigler-Najjar syndrome type 1. CPT Pharmacomet. Syst. Pharmacol. 7, 404–412 (2018).
Balakrishnan, B. et al. Novel mRNA-based therapy reduces toxic galactose metabolites and overcomes galactose sensitivity in a mouse model of classic galactosemia. Mol. Ther. 28, 304–312 (2020).
Karadagi, A. et al. Systemic modified messenger RNA for replacement therapy in alpha 1-antitrypsin deficiency. Sci. Rep. 10, 7052 (2020).
DeRosa, F. et al. Improved efficacy in a Fabry disease model using a systemic mRNA liver depot system as compared to enzyme replacement therapy. Mol. Ther. 27, 878–889 (2019).
Jiang, L. et al. Dual mRNA therapy restores metabolic function in long-term studies in mice with propionic acidemia. Nat. Commun. 11, 5339 (2020).
Cao, J. et al. mRNA therapy improves metabolic and behavioral abnormalities in a murine model of citrin deficiency. Mol. Ther. 27, 1242–1251 (2019).
Prieve, M. G. et al. Targeted mRNA therapy for ornithine transcarbamylase deficiency. Mol. Ther. 26, 801–813 (2018).
Wei, G. et al. Synthetic human ABCB4 mRNA therapy rescues severe liver disease phenotype in a BALB/c.Abcb4-/- mouse model of PFIC3. J. Hepatol. 74, 1416–1428 (2020).
Truong, B. et al. Lipid nanoparticle-targeted mRNA therapy as a treatment for the inherited metabolic liver disorder arginase deficiency. Proc. Natl Acad. Sci. USA 116, 21150–21159 (2019).
Yang, T. et al. Therapeutic HNF4A mRNA attenuates liver fibrosis in a preclinical model. J. Hepatol. 75, 1420–1433 (2021).
Van Hoecke, L. & Roose, K. How mRNA therapeutics are entering the monoclonal antibody field. J. Transl. Med. 17, 54 (2019).
Schlake, T., Thess, A., Thran, M. & Jordan, I. mRNA as novel technology for passive immunotherapy. Cell. Mol. Life Sci. 76, 301–328 (2019).
Schlake, T. et al. mRNA: a novel avenue to antibody therapy? Mol. Ther. 27, 773–784 (2019).
Yarnall, M. T. N. et al. Drag-and-drop genome insertion of large sequences without double-strand DNA cleavage using CRISPR-directed integrases. Nat. Biotechnol. 41, 500–512 (2023).
Durrant, M. G. et al. Systematic discovery of recombinases for efficient integration of large DNA sequences into the human genome. Nat. Biotechnol. 41, 488–499 (2023).
Mali, P. et al. RNA-guided human genome engineering via Cas9. Science 339, 823–826 (2013).
Cong, L. et al. Multiplex genome engineering using CRISPR/Cas systems. Science 339, 819–823 (2013).
McHugh, J. Acute inflammatory arthritis: long-term effects of chikungunya. Nat. Rev. Rheumatol. 14, 62 (2018).
Kose, N. et al. A lipid-encapsulated mRNA encoding a potently neutralizing human monoclonal antibody protects against chikungunya infection. Sci. Immunol. 4, eaaw6647 (2019).
Moderna. Moderna reports third quarter fiscal year 2021 financial results and provides business updates. Moderna https://investors.modernatx.com/news/news-details/2021/Moderna-Reports-Third-Quarter-Fiscal-Year-2021-Financial-Results-and-Provides-Business-Updates/default.aspx (2021).
Lundberg, A. M. et al. Key differences in TLR3/poly I:C signaling and cytokine induction by human primary cells: a phenomenon absent from murine cell systems. Blood 110, 3245–3252 (2007).
Karikó, K., Ni, H., Capodici, J., Lamphier, M. & Weissman, D. mRNA is an endogenous ligand for Toll-like receptor 3. J. Biol. Chem. 279, 12542–12550 (2004).
Thess, A. et al. Sequence-engineered mRNA without chemical nucleoside modifications enables an effective protein therapy in large animals. Mol. Ther. 23, 1456–1464 (2015).
Kauffman, K. J. et al. Efficacy and immunogenicity of unmodified and pseudouridine-modified mRNA delivered systemically with lipid nanoparticles in vivo. Biomaterials 109, 78–87 (2016).
Kalnin, K. V. et al. Immunogenicity and efficacy of mRNA COVID-19 vaccine MRT5500 in preclinical animal models. NPJ Vaccines 6, 61 (2021).
Chivukula, S. et al. Development of multivalent mRNA vaccine candidates for seasonal or pandemic influenza. NPJ Vaccines 6, 153 (2021).
Corleis, B. et al. Efficacy of an unmodified bivalent mRNA vaccine against SARS-CoV-2 variants in female small animal models. Nat. Commun. 14, 816 (2023).
Lutz, J. et al. Unmodified mRNA in LNPs constitutes a competitive technology for prophylactic vaccines. NPJ Vaccines 2, 29 (2017).
Lowe, D. CureVac comes around. Science: In the Pipeline https://www.science.org/content/blog-post/curevac-comes-around (2023).
Lenart, K. et al. A third dose of the unmodified COVID-19 mRNA vaccine CVnCoV enhances quality and quantity of immune responses. Mol. Ther. Methods Clin. Dev. 27, 309–323 (2022).
Sanofi. Sanofi announces positive phase 1/2 study interim results for its first mRNA-based vaccine candidate. Sanofi https://www.sanofi.com/en/media-room/press-releases/2021/2021-09-28-06-00-00-2304069 (2021).
Aldrich, C. et al. Proof-of-concept of a low-dose unmodified mRNA-based rabies vaccine formulated with lipid nanoparticles in human volunteers: a phase 1 trial. Vaccine 39, 1310–1318 (2021).
Callaway, E. & Naddaf, M. Pioneers of mRNA COVID vaccines win medicine Nobel. Nature https://doi.org/10.1038/d41586-023-03046-x (2023).
Generation Bio. Generation Bio provides update on preclinical studies for hemophilia a program. Generation Bio https://generationbio.com/news/generation-bio-provides-update-on-preclinical-studies-for-hemophilia-a-program/ (2021).
Lam, K. et al. Optimizing lipid nanoparticles for delivery in primates. Adv. Mater. 35, 2211420 (2023). This article provides a most welcome and valuable systematic evaluation of LNP performance and parameter optimization for NHPs.
Hatit, M. Z. C. et al. Species-dependent in vivo mRNA delivery and cellular responses to nanoparticles. Nat. Nanotechnol. 17, 310–318 (2022).
Besin, G. et al. Accelerated blood clearance of lipid nanoparticles entails a biphasic humoral response of B-1 followed by B-2 lymphocytes to distinct antigenic moieties. Immunohorizons 3, 282–293 (2019).
Suzuki, T. et al. Influence of dose and animal species on accelerated blood clearance of PEGylated liposomal doxorubicin. Int. J. Pharm. 476, 205–212 (2014).
Chen, E. et al. Premature drug release from polyethylene glycol (PEG)-coated liposomal doxorubicin via formation of the membrane attack complex. ACS Nano 14, 7808–7822 (2020).
Liu, S., Ishida, T. & Kiwada, H. Effect of serum components from different species on destabilizing hydrogenated phosphatidylcholine-based liposomes. Biol. Pharm. Bull. 20, 874–880 (1997).
Harashima, H. et al. Species difference in the disposition of liposomes among mice, rats, and rabbits: allometric relationship and species dependent hepatic uptake mechanism. Pharm. Res. 13, 1049–1054 (1996).
Yin, W. et al. Plasma lipid profiling across species for the identification of optimal animal models of human dyslipidemia. J. Lipid Res. 53, 51–65 (2012).
Elston, D. M. Survivorship bias. J. Am. Acad. Dermatol. https://doi.org/10.1016/j.jaad.2021.06.845 (2021).
Hemprich-Bennett, D., Rabaiotti, D. & Kennedy, E. Beware survivorship bias in advice on science careers. Nature https://doi.org/10.1038/d41586-021-02634-z (2021).
Stein, C. A. Keeping the biotechnology of antisense in context. Nat. Biotechnol. 17, 209 (1999).
Paunovska, K. et al. A direct comparison of in vitro and in vivo nucleic acid delivery mediated by hundreds of nanoparticles reveals a weak correlation. Nano Lett. 18, 2148–2157 (2018).
Whitehead, K. A. et al. In vitro–in vivo translation of lipid nanoparticles for hepatocellular siRNA delivery. ACS Nano 6, 6922–6929 (2012).
Kauffman, K. J. et al. Optimization of lipid nanoparticle formulations for mRNA delivery in vivo with fractional factorial and definitive screening designs. Nano Lett. 15, 7300–7306 (2015).
Guimaraes, P. P. G. et al. Ionizable lipid nanoparticles encapsulating barcoded mRNA for accelerated in vivo delivery screening. J. Control. Rel. 316, 404–417 (2019).
Dahlman, J. E. et al. Barcoded nanoparticles for high throughput in vivo discovery of targeted therapeutics. Proc. Natl Acad. Sci. USA 114, 2060–2065 (2017). In this article, a pooled approach for LNP screening is described, offering a new path for LNP discovery, potentially accelerating identification of novel LNPs with different tropisms.
Rhym, L. H., Manan, R. S., Koller, A., Stephanie, G. & Anderson, D. G. Peptide-encoding mRNA barcodes for the high-throughput in vivo screening of libraries of lipid nanoparticles for mRNA delivery. Nat. Biomed. Eng. 7, 901–910 (2023).
Sago, C. D. et al. High-throughput in vivo screen of functional mRNA delivery identifies nanoparticles for endothelial cell gene editing. Proc. Natl Acad. Sci. USA 115, E9944–E9952 (2018).
Suzuki, Y. et al. Biodegradable lipid nanoparticles induce a prolonged RNA interference-mediated protein knockdown and show rapid hepatic clearance in mice and nonhuman primates. Int. J. Pharm. 519, 34–43 (2017).
Ämmälä, C. et al. Targeted delivery of antisense oligonucleotides to pancreatic β-cells. Sci. Adv. 4, eaat3386 (2018).
Jafar-Nejad, P. et al. The atlas of RNase H antisense oligonucleotide distribution and activity in the CNS of rodents and non-human primates following central administration. Nucleic Acids Res. 49, 657–673 (2021).
Klose, A. D. & Paragas, N. Automated quantification of bioluminescence images. Nat. Commun. 9, 4262 (2018).
Kauffman, K. J. et al. Rapid, single-cell analysis and discovery of vectored mRNA transfection in vivo with a loxP-Flanked tdTomato reporter mouse. Mol. Ther. Nucleic Acids 10, 55–63 (2018).
Moreno-Mateos, M. A. et al. CRISPRscan: designing highly efficient sgRNAs for CRISPR-Cas9 targeting in vivo. Nat. Methods 12, 982–988 (2015).
Wang, T., Wei, J. J., Sabatini, D. M. & Lander, E. S. Genetic screens in human cells using the CRISPR-Cas9 system. Science 343, 80–84 (2014).
Crooke, S. T., Baker, B. F., Crooke, R. M. & Liang, X.-H. Antisense technology: an overview and prospectus. Nat. Rev. Drug Discov. 20, 427–453 (2021).
Holgersen, E. M. et al. Transcriptome-wide off-target effects of steric-blocking oligonucleotides. Nucleic Acid Ther. 31, 392–403 (2021).
Manghwar, H. et al. CRISPR/Cas systems in genome editing: methodologies and tools for sgRNA design, off-target evaluation, and strategies to mitigate off-target effects. Adv. Sci. 7, 1902312 (2020).
Wayment-Steele, H. K. et al. Theoretical basis for stabilizing messenger RNA through secondary structure design. Nucleic Acids Res. 49, 10604–10617 (2021).
Truebel, H. & Seidler, M. Mitigating bias in pharmaceutical R&D decision-making. Nat. Rev. Drug Discov. 21, 874–875 (2022).
Hon, C.-C. et al. An atlas of human long non-coding RNAs with accurate 5′ ends. Nature 543, 199–204 (2017).
Ren, F., Zhang, N., Zhang, L., Miller, E. & Pu, J. J. Alternative polyadenylation: a new frontier in post transcriptional regulation. Biomark. Res. 8, 67 (2020).
Liang, X.-H., Nichols, J. G., Sun, H. & Crooke, S. T. Translation can affect the antisense activity of RNase H1-dependent oligonucleotides targeting mRNAs. Nucleic Acids Res. 46, 293–313 (2018).
Gagnon, K. T. & Corey, D. R. Guidelines for experiments using antisense oligonucleotides and double-stranded RNAs. Nucleic Acid Ther. 29, 116–122 (2019). This paper is mandatory reading for everyone involved in RNA therapeutic discovery.
Wang, F. et al. G-rich motifs within phosphorothioate-based antisense oligonucleotides (ASOs) drive activation of FXN expression through indirect effects. Nucleic Acids Res. 50, 12657–12673 (2022).
Kleinman, M. E. et al. Sequence- and target-independent angiogenesis suppression by siRNA via TLR3. Nature 452, 591–597 (2008).
Putzbach, W. et al. Many si/shRNAs can kill cancer cells by targeting multiple survival genes through an off-target mechanism. eLife 6, e29702 (2017).
Whither RNAi? Nat. Cell Biol. 5, 489–490 (2003).
Hogrefe, R. I. An antisense oligonucleotide primer. Antisense Nucleic Acid Drug Dev. 9, 351–357 (1999).
Stein, C. A. The experimental use of antisense oligonucleotides: a guide for the perplexed. J. Clin. Invest. 108, 641–644 (2001).
Myers, K. J. & Dean, N. M. Sensible use of antisense: how to use oligonucleotides as research tools. Trends Pharmacol. Sci. 21, 19–23 (2000).
Crooke, S. T. Evaluating the mechanism of action of antiproliferative antisense drugs. Antisense Nucleic Acid Drug Dev. 10, 123–126 (2000).
Eckstein, F. et al. On the quality control of antisense oligonucleotides. Antisense Nucleic Acid Drug Dev. 6, 149 (1996).
Crooke, S. T. Proof of mechanism of antisense drugs. Antisense Nucleic Acid Drug Dev. 6, 145–147 (1996).
Stein, C. A. & Krieg, A. M. Problems in interpretation of data derived from in vitro and in vivo use of antisense oligodeoxynucleotides. Antisense Res. Dev. 4, 67–69 (1994).
Mattick, J. S. et al. Long non-coding RNAs: definitions, functions, challenges and recommendations. Nat. Rev. Mol. Cell Biol. 24, 430–447 (2023).
Clark, K. L. et al. Pharmacological characterization of a novel ENaCα siRNA (GSK2225745) with potential for the treatment of cystic fibrosis. Mol. Ther. Nucleic Acids 2, e65 (2013).
Androsavich, J. R. Assessing anti-miR pharmacology with miRNA polysome shift assay. Methods Mol. Biol. 1517, 103–113 (2017).
Lagarde, J. et al. High-throughput annotation of full-length long noncoding RNAs with capture long-read sequencing. Nat. Genet. 49, 1731–1740 (2017).
van Dongen, S., Abreu-Goodger, C. & Enright, A. J. Detecting microRNA binding and siRNA off-target effects from expression data. Nat. Methods 5, 1023–1025 (2008).
Scharner, J. et al. Hybridization-mediated off-target effects of splice-switching antisense oligonucleotides. Nucleic Acids Res. 48, 802–816 (2020).
Bartoszewski, R. et al. Codon bias and the folding dynamics of the cystic fibrosis transmembrane conductance regulator. Cell. Mol. Biol. Lett. 21, 23 (2016).
Olexiouk, V. et al. sORFs.org: a repository of small ORFs identified by ribosome profiling. Nucleic Acids Res. 44, D324–D329 (2016).
Liu, C.-X. & Chen, L.-L. Circular RNAs: characterization, cellular roles, and applications. Cell 185, 2016–2034 (2022).
Chen, Y. G. et al. Sensing self and foreign circular RNAs by intron identity. Mol. Cell 67, 228–238.e5 (2017).
Center for Biologics Evaluation & Research. Human gene therapy products incorporating human genome editing. US Food and Drug Administration https://www.fda.gov/regulatory-information/search-fda-guidance-documents/human-gene-therapy-products-incorporating-human-genome-editing (2021).
Lei, Z., Meng, H., Zhuang, Y., Zhu, Q. & Yi, C. Chemical and biological approaches to interrogate off-target effects of genome editing tools. ACS Chem. Biol. 18, 205–217 (2023).
Hunt, J. M. T., Samson, C. A., Rand, Adu & Sheppard, H. M. Unintended CRISPR-Cas9 editing outcomes: a review of the detection and prevalence of structural variants generated by gene-editing in human cells. Hum. Genet. 142, 705–720 (2023).
Scannell, J. W. & Bosley, J. When quality beats quantity: decision theory, drug discovery, and the reproducibility crisis. PLoS ONE 11, e0147215 (2016).
Altshuler, D. Vertex pharmaceuticals: humanizing drug discovery. https://www.nature.com/articles/d42473-020-00303-9 (2018). In this insightful feature piece, the author describes Vertex’s ‘next generation’ approach to drug development, which seeks to frontload investment into developing more candidates to lower the risk of late-stage attrition.
Mulligan, M. J. et al. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature 586, 589–593 (2020).
ClinicalTrials.gov. A trial investigating the safety and effects of four BNT162 vaccines against COVID-2019 in healthy and immunocompromised adults. ClinicalTrials.gov https://classic.clinicaltrials.gov/ct2/show/NCT04380701 (2022).
Anderson, B. A. et al. Towards next generation antisense oligonucleotides: mesylphosphoramidate modification improves therapeutic index and duration of effect of gapmer antisense oligonucleotides. Nucleic Acids Res. 49, 9026–9041 (2021).
Liu, J.-J. et al. CasX enzymes comprise a distinct family of RNA-guided genome editors. Nature 566, 218–223 (2019).
Foster, D. J. et al. Advanced siRNA designs further improve in vivo performance of GalNAc-siRNA conjugates. Mol. Ther. 26, 708–717 (2018).
Yamada, K. et al. Extended nucleic acid (exNA): a novel, biologically compatible backbone that significantly enhances oligonucleotide efficacy in vivo. Preprint at bioRxiv https://doi.org/10.1101/2023.05.26.542506 (2023). This article is a recent example of an improved nucleic acid chemistry for siRNAs, indicating that there is still much more innovation to come in RNA medicinal chemistry.
Hendel, A. et al. Chemically modified guide RNAs enhance CRISPR-Cas genome editing in human primary cells. Nat. Biotechnol. 33, 985–989 (2015).
Alterman, J. F. et al. A divalent siRNA chemical scaffold for potent and sustained modulation of gene expression throughout the central nervous system. Nat. Biotechnol. 37, 884–894 (2019).
Aditham, A. et al. Chemically modified mocRNAs for highly efficient protein expression in mammalian cells. ACS Chem. Biol. 17, 3352–3366 (2022).
Molina, A. G. & Sanghvi, Y. S. Liquid-phase oligonucleotide synthesis: past, present, and future predictions. Curr. Protoc. Nucleic Acid Chem. 77, e82 (2019).
Paul, S. et al. Convergent biocatalytic mediated synthesis of siRNA. ACS Chem. Biol. 10, 2183–2187 (2023).
Rosa, S. S., Prazeres, D. M. F., Azevedo, A. M. & Marques, M. P. C. mRNA vaccines manufacturing: challenges and bottlenecks. Vaccine 39, 2190–2200 (2021).
Miller, J. B. et al. Non-viral CRISPR/Cas gene editing in vitro and in vivo enabled by synthetic nanoparticle co-delivery of Cas9 mRNA and sgRNA. Angew. Chem. Int. Ed. 56, 1059–1063 (2017).
Breda, L. et al. In vivo hematopoietic stem cell modification by mRNA delivery. Science 381, 436–443 (2023). This article reports an exciting preclinical example of in vivo editing outside the liver, with potential for treating sickle cell disease (without toxic preconditioning).
Winkle, M., El-Daly, S. M., Fabbri, M. & Calin, G. A. Noncoding RNA therapeutics — challenges and potential solutions. Nat. Rev. Drug Discov. 20, 629–651 (2021).
Lee, E. C. et al. Discovery and preclinical evaluation of anti-miR-17 oligonucleotide RGLS4326 for the treatment of polycystic kidney disease. Nat. Commun. 10, 4148 (2019). This article describes an exemplar anti-miRNA therapeutic discovery programme with robust preclinical validation.
van der Ree, M. H. et al. Safety, tolerability, and antiviral effect of RG-101 in patients with chronic hepatitis C: a phase 1B, double-blind, randomised controlled trial. Lancet 389, 709–717 (2017).
Bader, A. G. miR-34 — a microRNA replacement therapy is headed to the clinic. Front. Genet. 3, 120 (2012).
Abdelaal, A. M. et al. A first-in-class fully modified version of miR-34a with outstanding stability, activity, and anti-tumor efficacy. Oncogene 42, 2985–2999 (2023).
Segal, M. et al. Hydrophobically modified let-7b miRNA enhances biodistribution to NSCLC and downregulates HMGA2 in vivo. Mol. Ther. Nucleic Acids 19, 267–277 (2020).
Pallan, P. S. et al. Structure and nuclease resistance of 2′,4′-constrained 2′-O-methoxyethyl (cMOE) and 2′-O-ethyl (cEt) modified DNAs. Chem. Commun. 48, 8195–8197 (2012).
Campbell, M. A. & Wengel, J. Locked vs. unlocked nucleic acids (LNA vs. UNA): contrasting structures work towards common therapeutic goals. Chem. Soc. Rev. 40, 5680–5689 (2011).
Despic, V. & Jaffrey, S. R. mRNA ageing shapes the Cap2 methylome in mammalian mRNA. Nature 614, 358–366 (2023).
Acknowledgements
The author expresses gratitude to D. Morrissey, M. Tadin-Strapps, M. Stewart, A. Barry and Y. Fu from Pfizer RNA Accelerator, as well as many other past and present colleagues, for their insights and helpful discussions that contributed to the creation of this manuscript. Biorender.com was used under paid subscription for initial figure creation; figures were redrawn by the journal for publication.
Author information
Authors and Affiliations
Contributions
J.R.A. contributed to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
J.R.A. was an employee and shareholder of Pfizer, Inc. at the time of this writing. He is an inventor on several patents related to RNA technologies and therapeutics.
Peer review
Peer review information
Nature Reviews Drug Discovery thanks Andrea Kasinski, Dave Ecker and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Adeno-associated virus
-
(AAV). A small, non-pathogenic virus commonly used as a gene delivery vector in gene therapy, with a limited cargo capacity of approximately 4.7 to 5.0 kilobases.
- Batten disease
-
A rare genetic disorder, also known as neuronal ceroid lipofuscinoses, caused by mutations in 1 of 13 genes that causes progressive neurological deterioration in children, leading to cognitive decline, seizures and loss of motor skills.
- Cap analysis of gene expression
-
(CAGE). A method for identifying transcriptional start sites and the identity of 5′ ends of mRNA at single-base pair resolution using next-generation sequencing.
- Closed-ended DNA
-
A non-viral capsid-free DNA vector with covalently closed ends used for gene transfer.
- Duplex RNA
-
A molecule composed of two complementary RNA strands that have formed a double-stranded structure through base pairing.
- Endosomes
-
Membrane-bound cellular organelles involved in the sorting, processing and trafficking of molecules within the cell, particularly during endocytosis.
- Gapmer
-
An oligo designed for RNAase-H-based knockdown with a central DNA region flanked by modified nucleotides. Shorthand nomenclature is often used to designate the length (in nucleotides) of each segment and the modification used, for example, 5-10-5 methoxyethyl (MOE) gapmer.
- Gymnosis
-
Refers to the still incompletely understood phenomenon by which molecules are delivered into cells without the use of any delivery vehicles or carriers.
- Hereditary transthyretin amyloidosis
-
(hATTR). A heterogenous, multisystem condition caused by mutations in the transthyretin (TTR) gene leading to abnormal deposits of amyloid protein in the heart, nerves and other organs. hATTR has several phenotypes including cardiomyopathy (hATTR-CM) with symptoms of heart failure and enlarged heart, and peripheral neuropathy (hATTR-PN) experienced as pain, loss of sensation, or tingling in the hands and feet and damage to the digestive tract and other organs.
- Internal ribosomal entry sites
-
(IRES). A structured RNA element that allows for translation initiation in a 5′ cap-independent manner, which means that ribosomes can bind directly to the IRES element within an RNA and initiate translation at or near this site.
- Ligand conjugates
-
Consist of a ligand attached to another molecule, for example, an oligonucleotide, often used for targeted drug delivery or imaging purposes.
- Lipid nanoparticle
-
(LNP). Formulations used to deliver nucleic acid-based therapeutics into cells, providing a protective and stable environment for efficient delivery to target cells and enhancing intracellular release into the cytoplasm.
- Macropinocytosis
-
An actin-driven process in which cells take in extracellular fluid and its contents by forming vesicles at the cell surface.
- Multivalency
-
Refers to the ability of a molecule to have multiple functions by interacting with or expressing multiple targets simultaneously.
- Pattern recognition receptors
-
(PRRs). A class of proteins that play a crucial part in the innate immune system by identifying specific molecular patterns associated with pathogens, triggering an immune response.
- Peripheral blood mononuclear cells
-
(PBMCs). Prepared from (human) whole blood containing lymphocytes, monocytes and progenitor populations.
- Plug-and-play
-
Colloquial term for the theoretical pinnacle of drug platform performance in which new products can be swiftly developed using pre-established parameters.
- Rapid amplification of cDNA ends
-
(RACE). A molecular biology technique used to obtain the sequence at the ends of an RNA transcript using reverse-transcription PCR amplification.
- Ribonuclease H
-
(RNAse-H). An enzyme that catalyses the cleavage of RNA in RNA–DNA hybrids leading to the degradation of the RNA component.
- Scrambled controls
-
Oligos generated with random or altered nucleotide arrangements that are used as experimental controls in an attempt to assess the specificity and biological effects of the original test oligo.
- Shear stress
-
Refers to the force exerted by flowing blood on nanoparticles and cells that can impact surface interactions and cellular uptake.
- Solid-phase synthesis
-
An efficient, automated production method in which oligonucleotides are assembled on a solid support, typically through controlled addition of nucleotide building blocks in a stepwise fashion.
- Survivorship bias
-
Refers to the error that occurs when only successful or surviving individuals or entities are considered in an analysis, leading to an inaccurate representation of the entire population or sample.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Androsavich, J.R. Frameworks for transformational breakthroughs in RNA-based medicines. Nat Rev Drug Discov (2024). https://doi.org/10.1038/s41573-024-00943-2
Accepted:
Published:
DOI: https://doi.org/10.1038/s41573-024-00943-2