Abstract
Background
This study aims to investigate the role of endoplasmic reticulum stress (ER stress) in human dermal lymphatic endothelial cells (HDLECs) and lymphatic malformations (LMs) and its relationship with aerobic glycolysis and inflammation.
Methods
The proliferation and apoptosis of HDLECs were examined with lipopolysaccharide (LPS) treatment. ER stress-associated proteins and glycolysis-related markers were detected by western blot. Glycolysis indexes were detected by seahorse analysis and lactic acid production assay kits. Immunohistochemistry was used to reveal the ER stress state of lymphatic endothelial cells (LECs) in LMs.
Results
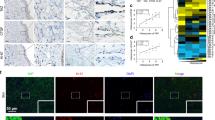
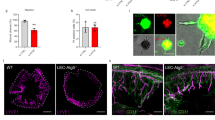
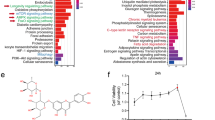
LPS induced ER stress in HDLECs but did not trigger detectable apoptosis. Intriguingly, LPS-treated HDLECs also showed increased glycolysis flux. Knockdown of Hexokinase 2, a key enzyme for aerobic glycolysis, significantly inhibited the ability of HDLECs to resist ER stress-induced apoptosis. Moreover, compared to normal skin, glucose-regulated protein 78 (GRP78/BIP), and phosphorylation protein kinase R-like kinase (p-PERK), two key ER stress-associated markers, were upregulated in LECs of LMs, which was correlated with the inflected state. In addition, excessively activated ER stress inhibited the progression of LMs in rat models.
Conclusions
These data indicate that glycolysis could rescue activated ER stress in HDLECs, which is required for the accelerated development of LMs.
Impact
-
Inflammation enhances both ER stress and glycolysis in LECs while glycolysis is required to attenuate the pro-apoptotic effect of ER stress.
-
Endoplasmic reticulum (ER) stress is activated in lymphatic endothelial cells (LECs) of LMs, especially in inflammatory condition.
-
The expression of ER stress-related proteins is increased in LMs and correlated with Hexokinase 2 expression.
-
Pharmacological activation of ER stress suppresses the formation of LM lesions in the rat model.
-
ER stress may be a promising and effective therapeutic target for the treatment of LMs.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The datasets used and/or analyses during the current study are available from the corresponding author on reasonable request.
References
Ma, W. & Oliver, G. Lymphatic endothelial cell plasticity in development and disease. Physiol. (Bethesda) 32, 444–452 (2017).
Jiang, H. et al. Pyruvate kinase M2 mediates glycolysis in the lymphatic endothelial cells and promotes the progression of lymphatic malformations. Am. J. Pathol. 191, 204–215 (2021).
Tammela, T. & Alitalo, K. Lymphangiogenesis: molecular mechanisms and future promise. Cell 140, 460–476 (2010).
Edwards, P. D., Rahbar, R., Ferraro, N. F., Burrows, P. E. & Mulliken, J. B. Lymphatic malformation of the lingual base and oral floor. Plast. Reconstructive Surg. 115, 1906–1915 (2005).
Elluru, R. G., Balakrishnan, K. & Padua, H. M. Lymphatic malformations: DiagNOSIS AND MANagement. Semin. Pediatr. Surg. 23, 178–185 (2014).
Walter, P. & Ron, D. The unfolded protein response: From stress pathway to homeostatic regulation. Science 334, 1081–1086 (2011).
Kozutsumi, Y., Segal, M., Normington, K., Gething, M. J. & Sambrook, J. The presence of malfolded proteins in the endoplasmic reticulum signals the induction of glucose-regulated proteins. Nature 332, 462–464 (1988).
McGuckin, M. A., Eri, R. D., Das, I., Lourie, R. & Florin, T. H. Er stress and the unfolded protein response in intestinal inflammation. Am. J. Physiol. Gastrointest. liver Physiol. 298, G820–G832 (2010).
Galán, M. et al. Mechanism of endoplasmic reticulum stress-induced vascular endothelial dysfunction. Biochimica et. biophysica acta 1843, 1063–1075 (2014).
Karali, E. et al. Vegf signals through Atf6 and perk to promote endothelial cell survival and angiogenesis in the absence of Er stress. Mol. Cell 54, 559–572 (2014).
Zeng, L. et al. Vascular endothelial cell growth-activated Xbp1 splicing in endothelial cells is crucial for angiogenesis. Circulation 127, 1712–1722 (2013).
Pereira, E. R., Liao, N., Neale, G. A. & Hendershot, L. M. Transcriptional and post-transcriptional regulation of proangiogenic factors by the unfolded protein response. PLoS One 5, e12521 (2010).
Ghosh, R. et al. Transcriptional regulation of Vegf-a by the unfolded protein response pathway. PLoS One 5, e9575 (2010).
Warburg, O. On the origin of cancer cells. Sci. (N. Y., NY) 123, 309–314 (1956).
Lunt, S. Y. & Vander Heiden, M. G. Aerobic glycolysis: Meeting the metabolic requirements of cell proliferation. Annu Rev. Cell Dev. Biol. 27, 441–464 (2011).
Pastorino, J. G. & Hoek, J. B. Hexokinase Ii: The integration of energy metabolism and control of apoptosis. Curr. medicinal Chem. 10, 1535–1551 (2003).
Chai, M. et al. Bleomycin restricts the glycolysis of lymphatic endothelial cells by inhibiting dimeric Pkm2 formation: A novel mechanism for lymphatic malformation treatment. Biochem Pharm. 204, 115227 (2022).
Zou, Y., Wang, R., Zhao, J., Cai, Y. & Zhong, W. Increased M2 isoform of pyruvate kinase in fibroblasts contributes to the growth, aggressiveness, and osteoclastogenesis of odontogenic keratocysts. Am. J. Pathol. 191, 857–871 (2021).
Zhang, W. et al. Infiltration of M2-polarized macrophages in infected lymphatic malformations: Possible role in disease progression. Br. J. Dermatol 175, 102–112 (2016).
Morris, M. C., Gilliam, E. A. & Li, L. Innate immune programing by endotoxin and its pathological consequences. Front Immunol. 5, 680 (2014).
Oyadomari, S. & Mori, M. Roles of Chop/Gadd153 in endoplasmic reticulum stress. Cell Death Differ. 11, 381–389 (2004).
Roberts, D. J. & Miyamoto, S. Hexokinase Ii integrates energy metabolism and cellular protection: Akting on mitochondria and torcing to autophagy. Cell Death Differ. 22, 248–257 (2015).
Fukunaga, M. Expression of D2-40 in lymphatic endothelium of normal tissues and in vascular tumours. Histopathology 46, 396–402 (2005).
Medzhitov, R. Origin and physiological roles of Inflammation. Nature 454, 428–435 (2008).
Wang, F. Q. et al. M2-polarised macrophages in infantile haemangiomas: correlation with promoted angiogenesis. J. Clin. Pathol. 66, 1058–1064 (2013).
Chae, B. S. Pretreatment of low-dose and super-low-dose Lps on the production of in vitro Lps-induced inflammatory mediators. Toxicol. Res. 34, 65–73 (2018).
Chen, K. et al. Super-low dose endotoxin pre-conditioning exacerbates sepsis mortality. EBioMedicine 2, 324–333 (2015).
Cao, S. S. & Kaufman, R. J. Unfolded protein response. Curr. Biol.: CB 22, R622–R626 (2012).
Nakayama, Y. et al. Molecular mechanisms of the Lps-induced non-apoptotic Er stress-chop pathway. J. Biochem 147, 471–483 (2010).
Li, W. et al. Crosstalk between Er stress, Nlrp3 Inflammasome, And Inflammation. Appl. Microbiol. Biotechnol. 104, 6129–6140 (2020).
Díaz-Bulnes, P., Saiz, M. L., López-Larrea, C. & Rodríguez, R. M. Crosstalk between hypoxia and Er stress response: A key regulator of macrophage polarization. Front Immunol. 10, 2951 (2019).
Duvigneau, J. C. et al. Crosstalk between inflammatory mediators and endoplasmic reticulum stress in liver diseases. Cytokine 124, 154577 (2019).
Blesinger, H. et al. Pik3ca mutations are specifically localized to lymphatic endothelial cells of lymphatic malformations. PLoS One 13, e0200343 (2018).
Li, L. J., Chai, Y., Guo, X. J., Chu, S. L. & Zhang, L. S. Effects of endoplasmic reticulum stress on autophagy and apoptosis of human leukemia cells via inhibition of the Pi3k/Akt/Mtor signaling pathway. Mol. Med. Rep. 17, 7886–7892 (2018).
Pang, X. L., He, G., Liu, Y. B., Wang, Y. & Zhang, B. Endoplasmic reticulum stress sensitizes human esophageal cancer cell to radiation. World J. Gastroenterol. 19, 1736–1748 (2013).
Park, S., Lim, W., Bazer, F. W. & Song, G. Naringenin induces mitochondria-mediated apoptosis and endoplasmic reticulum stress by regulating Mapk and Akt signal transduction pathways in endometriosis cells. Mol. Hum. Reprod. 23, 842–854 (2017).
Hetz, C. The unfolded protein response: Controlling cell fate decisions under Er stress and beyond. Nat. Rev. Mol. Cell Biol. 13, 89–102 (2012).
Yang, W. et al. Pkm2 phosphorylates histone H3 and promotes gene transcription and tumorigenesis. Cell 150, 685–696 (2012).
Funding
This study was funded by the National Natural Science Foundation of China (NSFC) grant 82370978, 81741082 (Y.C.), grant 81800994 (W.Z.), grant 81972548 (C.H.), National Health Commission of the People’s Republic of China grant 2019ZX09302011 (D.M. and Y.C.), Wuhan Science and Technology Bureau grant 2020020601012212 (Y. C.).
Author information
Authors and Affiliations
Contributions
W. Z., X. L. and H. J.: acquisition of data, analysis and interpretation of data; P. W.: interpretation of data; M. C., T. Z. and J. L.: analysis and interpretation of data. S. Y., D. M. and X. Z.: acquisition of data. C. H.: revising it critically for important intellectual conten; W. Z. and Y. C.: conception and design interpretation of data, revising it critically for important intellectual content and final approval of the version to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Statement of the patient consent
All the patients have signed the informed consent.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, X., Zhong, W., Jiang, H. et al. Endoplasmic reticulum stress is attenuated by glycolysis in lymphatic malformations. Pediatr Res (2024). https://doi.org/10.1038/s41390-024-03181-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41390-024-03181-9