
Ken-ichi Otake’s work focuses on the design and synthesis of new materials.Credit: Irwin Wong for Nature
This is the fifth in a Nature Index series of profiles about emerging early-careers researchers in Japan.
Chemistry has been part of Ken-ichi Otake’s life for as long as he can remember. As a boy, he frequently visited the lab of his father, an organic chemist who studied under Ryoji Noyori, one of Japan’s eight Nobel laureates in chemistry. “He would show me the instruments or just leave me to look around. I remember the place having an interesting smell,” says Otake.
His interest in the subject was rekindled when he entered Kyoto University as an undergraduate. “I remembered how much I loved chemistry. And I was influenced by my father, who was so proud of his work. He said that his job was his hobby,” says Otake, now an assistant professor at his alma mater’s Institute for Integrated Cell-Material Sciences (iCeMS).
Otake’s work focuses on the design and synthesis of new materials. He is especially interested in crystalline materials called porous coordination polymers (PCPs), also known as metal–organic frameworks. An example of such materials is the pigment Prussian blue, used to dye textiles and colour paints since the eighteenth century.
Interest in the polymers has seen a resurgence in the past 25 years as scientists have begun to synthesize new classes of PCPs that are extremely flexible.
The lattice structure of these novel PCPs, comprising of metal ions connected by organic molecules called linkers, resembles a kind of pliable playground climbing frame, with the open spaces acting as nano-sized pores or molecular gates that can be precisely controlled in response to external stimuli such as fluctuations in temperature.
“Because of this porous structure, PCPs have a very wide range of functionality,” says Otake. For instance, they can be used as catalysts and are also well-suited for separating compounds, especially small gases such as carbon monoxide, methane and hydrogen. That’s because scientists can design specialized polymers and control for characteristics such as pore size, he says.
Otake’s lab at Kyoto, led by Susumu Kitagawa, one of the first researchers to show how flexible PCPs could adsorb small molecules, is a global leader in using PCPs as a separation tool. In November 2022, the team, with collaborators in China, described a method for separating ‘heavy water’ (D2O) — water molecules containing the hydrogen isotope deuterium (D), also known as heavy hydrogen — from H2O (Y. Su et al. Nature 611, 289–302; 2022).
This was a breakthrough in the use of PCPs, says Otake. The water isotopologues D2O and H2O are notoriously hard to separate because they have near-identical melting and freezing points, molecular sizes and bond energies. Current approaches such as distillation and electrolysis are energy-intensive. “That’s why purifying heavy water is usually very expensive,” says Otake.
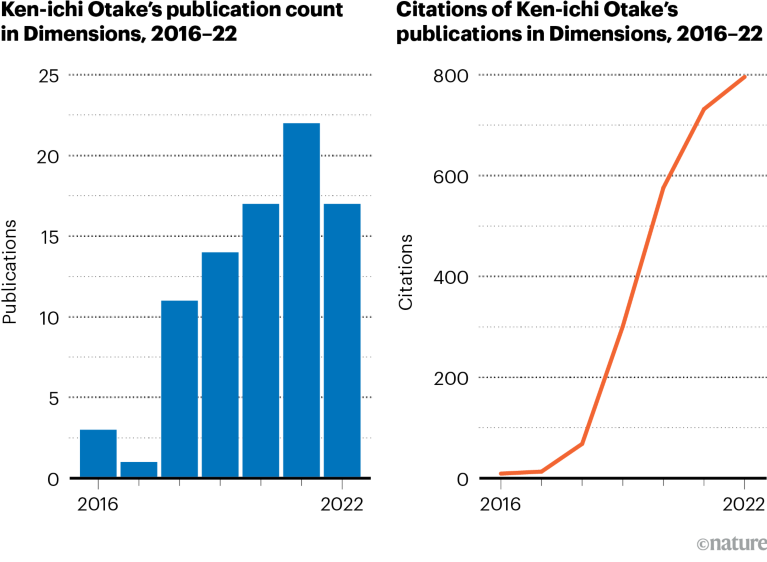
Source: Dimensions
D2O has extremely useful applications. It helps moderate reactions in nuclear power plants; increases vaccine thermostability and allows scientists to selectively label certain proteins, nucleic acids and other compounds, so that they may be tracked in real-time while undergoing various biological and chemical processes.
Otake and his team successfully separated heavy water from H2O by experimenting with two copper-based PCPs. At room temperature, the structure’s organic linkers ‘flip-flop’ open so that D2O and H2O — whose size and affinity closely matches that of the molecular gates — are adsorbed at different rates.
“By using this mechanism, we were able to separate water isotopologues using PCPs for the first time,” says Otake. The new approach could be far more efficient than current separation techniques if the method is developed into a practical solution that can put to everyday use, especially at scale.
The researchers are now exploring whether they can apply this to similar separation problems, including whether the technology can be used to remove carbon dioxide from factory flue gas. Otake is also keen to explore the “many other useful functions of porous materials”. He hopes his work will contribute to making new materials that are useful and energy efficient.
Otake was among the top five most-prolific early-career authors in Japan in the Nature Index for the field of chemistry from 2015 to 2021 — an accomplishment he says his father is proud of. He’s grateful to the Japanese government for boosting the funding for early-career researchers such as himself in recent years, and for the increase in non-tenured academic positions available.
Still, the number of graduates choosing to pursue PhDs or enter academia is waning, says Otake. Offering more long-term positions will help, but it’s just as important to “create an environment that allows researchers to focus on their work”, for instance, by providing financial assistance for day care, family care and personal health, he says.


 Is Japan’s research decline turning a corner?
Is Japan’s research decline turning a corner?
 How Japanese science is trying to reassert its research strength
How Japanese science is trying to reassert its research strength
 Japan’s leading international partnerships
Japan’s leading international partnerships
 Freeing up Japan’s PhD potential
Freeing up Japan’s PhD potential
 Japan’s rising research stars: Mariko Kimura
Japan’s rising research stars: Mariko Kimura
 Japan’s rising research stars: Yuuki Wada
Japan’s rising research stars: Yuuki Wada
 Japan’s rising research stars: Tatsuya Kubota
Japan’s rising research stars: Tatsuya Kubota
 Japan’s rising research stars: Yasuka Toda
Japan’s rising research stars: Yasuka Toda
 Will Japan’s new ¥10-trillion university fund lift research performance?
Will Japan’s new ¥10-trillion university fund lift research performance?
 Shoring up Japan’s research performance
Shoring up Japan’s research performance
 How Japan’s disciplinary strengths are shifting focus
How Japan’s disciplinary strengths are shifting focus
 Japanese robotics lags as AI captures global attention
Japanese robotics lags as AI captures global attention




