Abstract
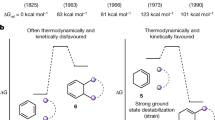
It is well established that strain in organic molecules is linked to having nonideal bond lengths, bond angles and unfavourable non-covalent interactions. The constrained geometries of ring systems are particularly predisposed to creating strain. Recently, there has been increased interest in leveraging this property of rings as a synthetic tool by building strain into substrates to activate a desired bond-cleavage step. However, one could also envisage alternative uses of strain. Here we outline how geometry control can be exploited to ‘switch on’ dynamic processes or stabilize reactive transition states. By designing constrained molecular structures that direct strain on particular bonds or functional groups, transformations that are otherwise energetically uphill can become favoured. This phenomenon can subvert our expectations about the reactivity and properties of organic molecules, giving rise to unusual bonding modes.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Gillespie, R. J. & Robinson, E. A. Electron domains and the VSEPR model of molecular geometry. Angew. Chem. Int. Ed. Engl. 35, 495–514 (1996).
McNaught, A. D. & Wilkinson, A. IUPAC Compendium of Chemical Terminology: The Gold Book (International Union of Pure and Applied Chemistry, 2006).
Fadler, R. E. & Flood, A. H. Rigidity and flexibility in rotaxanes and their relatives; on being stubborn and easy-going. Front. Chem. 10, 856173 (2022).
Deuter, J., Rodewald, H., Irngartinger, H., Loerzer, T. & Lüttke, W. Kristall-und Molekularstruktur von tetrakis(1-methylcyclopropyl)ethylen. Tetrahedron Lett. 26, 1031–1034 (1985).
Wiberg, K. B. The concept of strain in organic chemistry. Angew. Chem. Int. Ed. Engl. 25, 312–322 (1986).
Brown, F., Davies, T. D., Dostrovsky, I., Evans, O. J. & Hughes, E. D. Steric retardation and steric acceleration. Nature 167, 987–988 (1951).
Jiang, L. et al. Strain-driven formal [1,3]-aryl shift within molecular bows. Angew. Chem. Int. Ed. 62, e202312238 (2023).
Wilson, M. R. & Taylor, R. E. Strained alkenes in natural product synthesis. Angew. Chem. Int. Ed. 52, 4078–4087 (2013).
Sutthasupa, S., Shiotsuki, M. & Sanda, F. Recent advances in ring-opening metathesis polymerization, and application to synthesis of functional materials. Polym. J. 42, 905–915 (2010).
Spence, E. L., Langley, G. J. & Bugg, T. D. H. Cis-trans isomerization of a cyclopropyl radical trap catalyzed by extradiol catechol dioxygenases: evidence for a semiquinone intermediate. J. Am. Chem. Soc. 118, 8336–8343 (1996).
Sweet, R. M. & Dahl, L. F. Molecular architecture of the cephalosporins. Insights into biological activity based on structural investigations. J. Am. Chem. Soc. 92, 5489–5507 (1970).
Turkowska, J., Durka, J. & Gryko, D. Strain release—an old tool for new transformations. Chem. Commun. 56, 5718–5734 (2020).
Wittig, G. & Krebs, A. Zur Existenz niedergliedriger Cycloalkine, I. Chem. Ber. 94, 3260–3275 (1961).
Agard, N. J., Prescher, J. A. & Bertozzi, C. R. A strain-promoted [3+2] azide-alkyne cycloaddition for covalent modification of biomolecules in living systems. J. Am. Chem. Soc. 126, 15046–15047 (2004).
Gianatassio, R. et al. Strain-release amination. Science 351, 241–246 (2016).
Lopchuk, J. M. et al. Strain-release heteroatom functionalization: development, scope and stereospecificity. J. Am. Chem. Soc. 139, 3209–3226 (2017).
Shi, J., Li, L. & Li, Y. o-Silylaryl triflates: a journey of Kobayashi aryne precursors. Chem. Rev. 121, 3892–4044 (2021).
Kelleghan, A. V., Bulger, A. S., Witkowski, D. C. & Garg, N. K. Strain-promoted reactions of 1,2,3-cyclohexatriene and its derivatives. Nature 618, 748–754 (2023).
Novianti, I., Kowada, T. & Mizukami, S. Clip to click: controlling inverse electron-demand Diels-Alder reactions with macrocyclic tetrazines. Org. Lett. 24, 3223–3226 (2022).
Moteki, M., Maeda, S. & Ohno, K. Systematic search for isomerization pathways of hexasilabenzene for finding its kinetic stability. Organometallics 28, 2218–2224 (2009).
Jensen, F. R. et al. Separation of conformers. II. Axial and equatorial isomers of chlorocyclohexane and trideuteriomethoxycyclohexane. J. Am. Chem. Soc. 91, 3223–3225 (1969).
Prelog, V. & Wieland, P. Über die Spaltung der Tröger’schen Base in optische Antipoden, ein Beitrag zur Stereochemie des dreiwertigen Stickstoffs. Helv. Chim. Acta 27, 1127–1134 (1944).
Brunel, J. M. BINOL: a versatile chiral reagent. Chem. Rev. 105, 857–897 (2005).
Rössler, S. L., Petrone, D. A. & Carreira, E. M. Iridium-catalyzed asymmetric synthesis of functionally rich molecules enabled by (phosphoramidite,olefin) ligands. Acc. Chem. Res. 52, 2657–2672 (2019).
Smith, O. et al. Control of stereogenic oxygen in a helically chiral oxonium ion. Nature 615, 430–435 (2023).
Tang, M. et al. Molecular-strain engineering of double-walled tetrahedra. Chem 7, 2160–2174 (2021).
Wu, L. et al. Synthesis of contra-helical trefoil knots with mechanically tuneable spin-crossover properties. Nat. Synth. 2, 17–25 (2023).
Tang, M. et al. Mechanical trapping and in situ derivatization of the porphodimethene intermediate. Mater. Today Chem. 24, 100868 (2022).
Tang, M., Liang, Y., Liu, J., Bian, L. & Liu, Z. Mechanical trapping of the phlorin intermediate. CCS Chem. 4, 3230–3237 (2022).
Saha, P. K. et al. Rupturing aromaticity by periphery overcrowding. Nat. Chem. 15, 516–525 (2023).
Bismillah, A. N. et al. Control of dynamic sp3-C stereochemistry. Nat. Chem. 15, 615–624 (2023).
Schreiner, P. R. Quantum mechanical tunneling is essential to understanding chemical reactivity. Trends Chem. 2, 980–989 (2020).
Castro, C. & Karney, W. L. Heavy-atom tunneling in organic reactions. Angew. Chem. Int. Ed. 59, 8355–8366 (2020).
Tran Ngoc, T., Grabicki, N., Irran, E., Dumele, O. & Teichert, J. F. Photoswitching neutral homoaromatic hydrocarbons. Nat. Chem. 15, 377–385 (2023).
Aricó, F. et al. Templated synthesis of interlocked molecules. Top. Curr. Chem. 249, 203–259 (2005).
Beves, J. E., Blight, B. A., Campbell, C. J., Leigh, D. A. & McBurney, R. T. Strategies and tactics for the metal-directed synthesis of rotaxanes, knots, catenanes and higher order links. Angew. Chem. Int. Ed. 50, 9260–9327 (2011).
Martí-Centelles, V., Pandey, M. D., Burguete, M. I. & Luis, S. V. Macrocyclization reactions: the importance of conformational, configurational and template-induced preorganization. Chem. Rev. 115, 8736–8834 (2015).
Galan, A. & Ballester, P. Stabilization of reactive species by supramolecular encapsulation. Chem. Soc. Rev. 45, 1720–1737 (2016).
Feringa, B. L. & Browne, W. R. Molecular Switches 2nd edn (Wiley, 2011).
Pianowski, Z. L. Molecular Photoswitches: Chemistry, Properties and Applications Vol. 2 (Wiley, 2022).
Jung, M. E. Substituent and solvent effects in intramolecular Diels-Alder reactions. Synlett 1990, 186–190 (1990).
McNamara, O. A. & Maguire, A. R. The norcaradiene-cycloheptatriene equilibrium. Tetrahedron 67, 9–40 (2011).
Vogel, E. Valenzisomerisierungen von Verbindungen mit gespannten Ringen. Angew. Chem. 74, 829–839 (1962).
Schreiber, J. et al. A. Synthese des Colchicins. Helv. Chim. Acta 44, 540–597 (1961).
Vogel, E., Wiedemann, W., Roth, H. D., Eimer, J. & Günther, H. Ein Beitrag zum Norcaradien–Cycloheptatrien-Strukturproblem. Justus Liebigs Ann. Chem. 759, 1–36 (1972).
Vogel, E., Wiedemann, W., Kiefer, H. & Harrison, W. F. Über das Norcaradien- und das Vinyloge Bicyclo[6.1.0]nona-2.4.6-trien-system. Tetrahedron Lett. 4, 673–678 (1963).
Berlin, L. et al. Offenkettige und Cyclische Polyene, En-ine (Georg Thieme, 1972).
Cope, A. C. & Hardy, E. M. The introduction of substituted vinyl groups. V. A rearrangement involving the migration of an allyl group in a three-carbon system. J. Am. Chem. Soc. 62, 441–444 (1940).
Olson, J. A. & Shea, K. M. Critical perspective: named reactions discovered and developed by women. Acc. Chem. Res. 44, 311–321 (2011).
Schneider, M. P. & Ran, A. Synthesis and Cope rearrangement of cis-1,2-dialkenylcyclopropanes. J. Am. Chem. Soc. 101, 4426–4427 (1979).
Graulich, N. The Cope rearrangement—the first born of a great family. Wiley Interdiscip. Rev. Comput. Mol. Sci. 1, 172–190 (2011).
von Eggers Doering, W. et al. A rational synthesis of bullvalene barbaralone and derivatives; bullvalone. Tetrahedron 23, 3943–3963 (1967).
Günther, H., Runsink, J., Schmickler, H. & Schmitt, P. Applications of 13C NMR spectroscopy. 26. Activation parameters for the degenerate Cope rearrangement of barbaralane and 3,7-disubstituted barbaralanes. J. Org. Chem. 50, 289–293 (1985).
Cheng, A. K., Anet, F. A. L., Mioduski, J. & Meinwald, J. Determination of the fluxional barrier in semibullvalene by proton and carbon-13 nuclear magnetic resonance spectroscopy. J. Am. Chem. Soc. 96, 2887–2891 (1974).
Saunders, M. Measurement of the rate of rearrangement of bullvalene. Tetrahedron Lett. 4, 1699–1702 (1963).
Williams, R. V. Semibullvalenes and related molecules: ever closer approaches to neutral homoaromaticity. Eur. J. Org. Chem. 2001, 227–235 (2001).
Anet, F. A. L. & Schenck, G. E. Temperature-dependent nuclear magnetic resonance spectrum of octamethylsemibullvalene. Tetrahedron Lett. 11, 4237–4240 (1970).
Lambert, J. B. The degenerate Cope rearrangement of tricyclo[3.3.1.04,6]nona-2,7-diene-9-one. Tetrahedron Lett. 4, 1901–1906 (1963).
Moskau, D. et al. Anwendungen der 13C-NMR-spektroskopie, XXVII. Die Aktivierungsparameter der Cope-Umlagerung von Semibullvalen, 1,5-dimethylsemibullvalen und 2,6-dibrom-1,5-dimethylsemibullvalen. Chem. Ber. 122, 925–931 (1989).
Allerhand, A. & Gutowsky, H. S. Spin-echo nuclear magnetic resonance studies of chemical exchange. VI. Rearrangement of bullvalene and of its silver nitrate complex. J. Am. Chem. Soc. 87, 4092–4096 (1965).
Williams, R. V., Al-Sehemi, A. G., Meier, A. K., Brown, Z. Z. & Armantrout, J. R. The role of strain in the homoaromatization of semibullvalenes. J. Org. Chem. 82, 4136–4147 (2017).
Zhang, X., Hrovat, D. A. & Borden, W. T. Calculations predict that carbon tunneling allows the degenerate Cope rearrangement of semibullvalene to occur rapidly at cryogenic temperatures. Org. Lett. 12, 2798–2801 (2010).
Schleif, T. et al. Heavy-atom tunneling in semibullvalenes: how driving force, substituents, and environment influence the tunneling rates. Chem. Eur. J. 26, 10452–10458 (2020).
Schleif, T. et al. The Cope rearrangement of 1,5-dimethylsemibullvalene-2(4)-d1: experimental evidence for heavy-atom tunneling. Angew. Chem. Int. Ed. 56, 10746–10749 (2017).
Williams, R. V. et al. 1,5-Dimethyl-2,4,6,8-semibullvalenetetracarboxylic dianhydride: a close approach to a neutral homoaromatic semibullvalene. J. Am. Chem. Soc. 118, 4208–4209 (1996).
Quast, H. & Seefelder, M. The equilibrium between localized and delocalized states of thermochromic semibullvalenes and barbaralanes—direct observation of transition states of degenerate Cope rearrangements. Angew. Chem. Int. Ed. 38, 1064–1067 (1999).
Seefelder, M. & Quast, H. Solvent effects on the equilibrium between localized and delocalized states of thermochromic semibullvalenes and barbaralanes. Angew. Chem. Int. Ed. 38, 1068–1071 (1999).
Bedi, A., Shimon, L. J. W. & Gidron, O. Helically locked tethered twistacenes. J. Am. Chem. Soc. 140, 8086–8090 (2018).
Bedi, A., Manor Armon, A. & Gidron, O. Effect of twisting on the capture and release of singlet oxygen by tethered twisted acenes. Org. Lett. 22, 7809–7813 (2020).
Bodwell, G. J., Bridson, J. N., Houghton, T. J., Kennedy, J. W. J. & Mannion, M. R. 1,7-Dioxa[7](2,7)pyrenophane: the pyrene moiety is more bent than that of C70. Chem. Eur. J. 5, 1823–1827 (1999).
Zhang, X., Mackinnon, M. R., Bodwell, G. J. & Ito, S. Synthesis of a π-extended azacorannulenophane enabled by strain-induced 1,3-dipolar cycloaddition. Angew. Chem. Int. Ed. 61, e202116585 (2022).
Grommet, A. B., Feller, M. & Klajn, R. Chemical reactivity under nanoconfinement. Nat. Nanotechnol. 15, 256–271 (2020).
Schramm, V. L. Transition states, analogues and drug development. ACS Chem. Biol. 8, 71–81 (2013).
Schramm, V. L. Transition states and transition state analogue interactions with enzymes. Acc. Chem. Res. 48, 1032–1039 (2015).
Evans, G. B., Schramm, V. L. & Tyler, P. C. The transition to magic bullets—transition state analogue drug design. MedChemComm 9, 1983–1993 (2018).
Day, A. C. Aromaticity and the generalized Woodward-Hoffmann rules for pericyclic reactions. J. Am. Chem. Soc. 97, 2431–2438 (1975).
Krygowski, T. M., Szatylowicz, H., Stasyuk, O. A., Dominikowska, J. & Palusiak, M. Aromaticity from the viewpoint of molecular geometry: application to planar systems. Chem. Rev. 114, 6383–6422 (2014).
Shaik, S. S., Hiberty, P. C., Lefour, J. M. & Ohanessian, G. Is delocalization a driving force in chemistry? Benzene, allyl radical, cyclobutadiene and their isoelectronic species. J. Am. Chem. Soc. 109, 363–374 (1987).
Castro, C., Karney, W. L., McShane, C. M. & Pemberton, R. P. [10]Annulene: bond shifting and conformational mechanisms for automerization. J. Org. Chem. 71, 3001–3006 (2006).
von Ragué Schleyer, P., Jiao, H., Sulzbach, H. M. & Schaefer, H. F. Highly aromatic planar all-cis-[10]annulene derivatives. J. Am. Chem. Soc. 118, 2093–2094 (1996).
Parmar, K., Blaquiere, C. S., Lukan, B. E., Gengler, S. N. & Gravel, M. Synthesis of a highly aromatic and planar dehydro [10]annulene derivative. Nat. Synth. 1, 696–700 (2022).
Bean, D. E. & Fowler, P. W. Effect on ring current of the Kekulé vibration in aromatic and antiaromatic rings. J. Phys. Chem. A 115, 13649–13656 (2011).
Bürgi, H. et al. X-ray diffraction evidence for a cyclohexatriene motif in the molecular structure of tris(bicyclo[2.1.1]hexeno)benzene: bond alternation after the refutation of the Mills-Nixon theory. Angew. Chem. Int. Ed. Engl. 34, 1454–1456 (1995).
Jenneskens, L. W. et al. Molecular structure of 8,11-dichloro[5]metacyclophane: a strongly bent benzene ring. Angew. Chem. Int. Ed. Engl. 23, 238–239 (1984).
Jenneskens, L. W. et al. [5]Paracyclophane. J. Am. Chem. Soc. 107, 3716–3717 (1985).
Matsuura, A. & Komatsu, K. Efficient synthesis of benzene and planar cyclooctatetraene fully annelated with bicyclo[2.1.1]hex-2-ene. J. Am. Chem. Soc. 123, 1768–1769 (2001).
Narsaria, A. K., Hamlin, T. A., Lammertsma, K. & Bickelhaupt, F. M. Dual activation of aromatic Diels-Alder reactions. Chem. Eur. J. 25, 9902–9912 (2019).
Jenneskens, L. W., de Boer, H. J. R., de Wolf, W. H. & Bickelhaupt, F. Reactivity of 8,11-dihalo[5]metacyclophanes. J. Am. Chem. Soc. 112, 8941–8949 (1990).
Li, L. et al. Stabilizing a different cyclooctatetraene stereoisomer. Proc. Natl Acad. Sci. USA 114, 9803–9808 (2017).
Baldridge, K. K. & Siegel, J. S. Quantum mechanical designs toward planar delocalized cyclooctatetraene: a new target for synthesis. J. Am. Chem. Soc. 123, 1755–1759 (2001).
Hashemi, M. M., Bratcher, M. S. & Scott, L. T. Corannulene bowl-to-bowl inversion is rapid at room temperature. J. Am. Chem. Soc. 114, 1920–1921 (1992).
Solel, E. et al. Flat corannulene: when a transition state becomes a stable molecule. Chem. Sci. 11, 13015–13025 (2020).
Martin, J. C. ‘Frozen’ transition states: pentavalent carbon et al. Science 221, 509–514 (1983).
Yamashita, M. et al. Syntheses and structures of hypervalent pentacoordinate carbon and boron compounds bearing an anthracene skeleton—elucidation of hypervalent interaction based on X-ray analysis and DFT calculation. J. Am. Chem. Soc. 127, 4354–4371 (2005).
Griffiths, P. R., Pivonka, D. E. & Williams, R. V. The experimental realization of a neutral homoaromatic carbocycle. Chem. Eur. J. 17, 9193–9199 (2011).
Williams, R. V. Homoaromaticity. Chem. Rev. 101, 1185–1204 (2001).
Vogel, E., Feldmann, R. & Düwel, H. Synthese des bicyclo[5.4.1]dodecapentaenylium-ions aus cycloheptatrien-1.6-dialdehyd. Tetrahedron Lett. 11, 1941–1944 (1970).
Paquette, L. A., Wingard, R. E. & Russell, R. K. Structural consequences of 2,8 bridging of the semibullvalene nucleus. J. Am. Chem. Soc. 94, 4739–4741 (1972).
Vogel, E., Brinker, U. H., Nachtkamp, K., Wassen, J. & Müllen, K. Semibullvalenes as potential homoaromatic compounds. Angew. Chem. Int. Ed. Engl. 12, 758–760 (1973).
Tran Ngoc, T., van Der Welle, J., Rüffer, T. & Teichert, J. F. Synthesis of stable neutral homoaromatic hydrocarbons. Synthesis 55, 2658–2669 (2023).
Colwell, C. E., Price, T. W., Stauch, T. & Jasti, R. Strain visualization for strained macrocycles. Chem. Sci. 11, 3923–3930 (2020).
Chen, Z. et al. Nucleus-independent chemical shifts (NICS) as an aromaticity criterion. Chem. Rev. 105, 3842–3888 (2005).
Geuenich, D., Hess, K., Köhler, F. & Herges, R. Anisotropy of the induced current density (ACID), a general method to quantify and visualize electronic delocalization. Chem. Rev. 105, 3758–3772 (2005).
Greer, E. M., Kwon, K., Greer, A. & Doubleday, C. Thermally activated tunneling in organic reactions. Tetrahedron 72, 7357–7373 (2016).
Nicolaou, K. C., Zuccarello, G., Riemer, C., Estevez, V. A. & Dai, W. M. Design, synthesis and study of simple monocyclic conjugated enediynes. The 10-membered ring enediyne moiety of the enediyne anticancer antibiotics. J. Am. Chem. Soc. 114, 7360–7371 (1992).
Suffert, J., Abraham, E., Raeppel, S. & Brückner, R. Synthesis of 5-/10-membered ring analogues of the eienediyne core of neocarzinostatine chromophore by palladium(0)-mediated ring-closure reaction. Liebigs Ann. 1996, 447–456 (1996).
Greer, E. M., Cosgriff, C. V. & Doubleday, C. Computational evidence for heavy-atom tunneling in the Bergman cyclization of a 10-membered-ring enediyne. J. Am. Chem. Soc. 135, 10194–10197 (2013).
Karmakar, S. & Datta, A. Role of heavy atom tunnelling in Myers-Saito cyclization of cyclic enyne-cumulene systems. J. Phys. Chem. B 120, 945–950 (2016).
Sanchez, A. & Maimone, T. J. Taming shapeshifting anions: total synthesis of ocellatusone C. J. Am. Chem. Soc. 144, 7594–7599 (2022).
Sanchez, A. et al. A shapeshifting roadmap for polycyclic skeletal evolution. J. Am. Chem. Soc. 145, 13452–13461 (2023).
Dewick, P. M. Medicinal Natural Products: A Biosynthetic Approach 3rd edn (Wiley, 2009).
Defieber, C., Grützmacher, H. & Carreira, E. M. Chiral olefins as steering ligands in asymmetric catalysis. Angew. Chem. Int. Ed. 47, 4482–4502 (2008).
Teichert, J. F. & Feringa, B. L. Phosphoramidites: privileged ligands in asymmetric catalysis. Angew. Chem. Int. Ed. 49, 2486–2528 (2010).
Tantillo, D. J. & Hoffmann, R. Snakes and ladders. The sigmatropic shiftamer concept. Acc. Chem. Res. 39, 477–486 (2006).
Tantillo, D. J. et al. Extended barbaralanes: sigmatropic shiftamers or σ-polyacenes? J. Am. Chem. Soc. 126, 4256–4263 (2004).
Bismillah, A. N., Chapin, B. M., Hussein, B. A. & McGonigal, P. R. Shapeshifting molecules: the story so far and the shape of things to come. Chem. Sci. 11, 324–332 (2020).
Pomfret, M. N. et al. Stochastic bullvalene architecture modulates structural rigidity in π-rich macromolecules. Angew. Chem. Int. Ed. 62, e202301695 (2023).
Yahiaoui, O., Pašteka, L. F., Judeel, B. & Fallon, T. Synthesis and analysis of substituted bullvalenes. Angew. Chem. Int. Ed. 57, 2570–2574 (2018).
Ottonello, A. et al. Shapeshifting bullvalene-linked vancomycin dimers as effective antibiotics against multidrug-resistant gram-positive bacteria. Proc. Natl Acad. Sci. USA 120, e2208737120 (2023).
Reimers, J. R. et al. Controlling piezoresistance in single molecules through the isomerisation of bullvalenes. Nat. Commun. 14, 6089 (2023).
Schröder, G., Oth, J. F. M. & Merényi, R. Molecules undergoing fast, reversible valence-bond isomerization. (Molecules with fluctuating bonds). Angew. Chem. Int. Ed. Engl. 4, 752–761 (1965).
Muller, P. Glossary of terms used in physical organic chemistry: (IUPAC recommendations 1994). Pure Appl. Chem. 66, 1077–1184 (1994).
Mitschke, B., Turberg, M. & List, B. Confinement as a unifying element in selective catalysis. Chem 6, 2515–2532 (2020).
Bürgi, H. B. & Dunitz, J. D. From crystal statics to chemical dynamics. Acc. Chem. Res. 16, 153–161 (1983).
Jana, D. F., Wodrich, M. D. & Corminboeuf, C. Structure-correlation principles connecting ground state properties and reaction barrier heights for the Cope rearrangement of semibullvalenes. J. Org. Chem. 77, 2548–2552 (2012).
Acknowledgements
P.K.S. gratefully acknowledges the Engineering and Physical Sciences Research Council (EPSRC) for a Doctoral Training Grant (EP/R513039/1). P.R.M. acknowledges a Leverhulme Trust Research Project Grant (RPG-2023-191) and an EPSRC Fellowship (EP/V040049/2).
Author information
Authors and Affiliations
Contributions
All authors conceived the main outline and idea of the Review and contributed to the writing. P.K.S. and T.T.N. prepared the figures.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks Ori Gidron and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Alison Stoddart, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Saha, P.K., Tran Ngoc, T., McGonigal, P.R. et al. Geometry-controlled reactivity and dynamics in organic molecules. Nat. Synth (2024). https://doi.org/10.1038/s44160-024-00526-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s44160-024-00526-4